Exhibit 99.20
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. This short form prospectus constitutes a public offering of these securities only in those jurisdictions where they may be lawfully offered for sale and therein only by the persons permitted to sell such securities.
The securities offered under this short form prospectus have not been and will not be registered under the United States Securities Act of 1933, as amended (the “U.S. Securities Act”), or the securities laws of any state of the United States (as such term is defined in Regulation S under the U.S. Securities Act) (the “United States”), and may not be offered or sold within the United States, or to, or for the account or benefit of a U.S. Person (as defined in Rule 902(k) of Regulation S under the U.S. Securities Act) or a person in the United States, except as permitted by the Underwriting Agreement (as defined herein) and in transactions exempt from registration under the U.S. Securities Act and applicable U.S. state securities laws. This short form prospectus does not constitute an offer to sell or a solicitation of an offer to buy any of the securities offered hereby within the United States or to, or for the account or benefit of, U.S. persons.
Information has been incorporated by reference in this short form prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of the Company at our head office located at 789 West Pender Street, Suite 810, Vancouver, BC, V6C
1H2, Telephone 604-687-2038, and are also available electronically at www.sedar.com.
New Issue September 18, 2020
SHORT FORM PROSPECTUS

RED WHITE & BLOOM BRANDS INC.
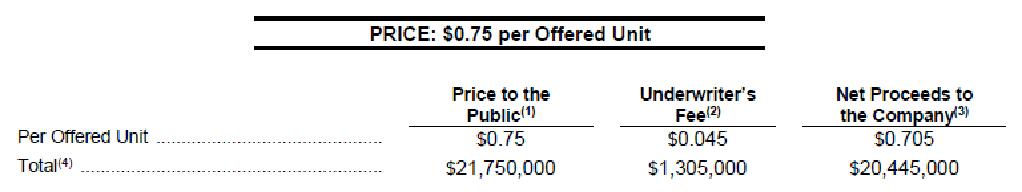
$21,750,000
29,000,000 UNITS
This short form prospectus (the “Prospectus”) qualifies the distribution and offering (the “Offering”) of 29,000,000 units (the “Offered Units”) of Red White & Bloom Brands Inc. (“RWB” or the “Company”) at a price of $0.75 per Offered Unit (the “Offering Price”) for total gross proceeds of $21,750,000, pursuant to the terms of an underwriting agreement (the “Underwriting Agreement”) dated August 25, 2020 between the Company, PI Financial Corp. and Eight Capital as co-lead underwriters (the “Co-Lead Underwriters”), together with Canaccord Genuity Corp. and Echelon Wealth Partners Inc. (collectively, the “Underwriters”), as amended. See “Plan of Distribution”.
Each Offered Unit consists of one common share (“Common Share”) in the capital of the Company (each a “Unit Share”) and one transferable Common Share purchase warrant of the Company (each such warrant, a “Warrant”). Each Warrant will entitle the holder thereof to acquire, subject to adjustment in certain circumstances, one Common Share (each, a “Warrant Share”) at an exercise price equal to $1.00 per Warrant Share (the “Exercise Price”) for a period of twenty-four (24) months following the Closing Date (as defined herein). If, at any time prior to the expiry date of the Warrants, the volume-weighted average price of the Common Shares on the Canadian Securities Exchange (the “CSE”) (or such other stock exchange where the majority of the trading volume occurs) exceeds
$1.50 for 10 consecutive trading days, the Company may provide written notice to the holders of the Warrants by way of a news release advising that the Warrants will expire at 5:00 p.m. (Vancouver time) on the 30th day following the date of such notice unless exercised by the holders prior to such date (the “Accelerated Exercise Period”). The Warrants will be governed by a warrant indenture (the “Warrant Indenture”) to be entered into on the Closing Date between the Company and National Securities Administrators Ltd., as warrant agent. See “Description of Securities Being Distributed”.
The Underwriters have agreed to act as, and the Company has appointed the Underwriters as, the sole and exclusive underwriters of the Company to offer the Offered Units for sale in accordance with the conditions contained in the Underwriting Agreement.
ii
The Common Shares are listed and posted for trading on the CSE under the trading symbol “RWB” and trade in the United States on the OTCQX under the trading symbol “RWBYF”. On September 17, 2020, the last trading day prior to the date of this Prospectus, the closing prices of the Common Shares listed on the CSE and the OTCQX were $0.61 and US$0.48, respectively.
The Company has applied to list the Unit Shares, the Warrants and the Warrant Shares (including those underlying the Over-Allotment Units (each as defined herein)), the Compensation Shares, the Compensation Warrants and the Compensation Warrant Shares (each as defined herein) on the CSE. Listing will be subject to the Company fulfilling all listing requirements of the CSE. There is currently no market through which the Warrants may be sold and purchasers may not be able to resell the Warrants purchased under this Prospectus. See “Risk Factors” and “Plan of Distribution”.
Notes:
(1) The Offering Price was determined by arm’s length negotiation between the Company and the Co-Lead Underwriters on behalf of the Underwriters, with reference to the prevailing market price of the Common Shares.
(2) The Company has agreed to pay to the Underwriters a cash fee equal to 6.0% of gross proceeds raised in respect of the Offering (including any gross proceeds raised on exercise of the Over-Allotment Option (as defined herein)) (collectively, the “Underwriters’ Fee”). As additional consideration for the services rendered in connection with the Offering, the Company has agreed to issue to the Underwriters such number of non-transferable compensation options (the “Compensation Options”) to acquire that number of units of the Company on the same terms as the Offered Units (the “Compensation Units”) as is equal to 6.0% of the number of Offered Units sold under the Offering (including upon the exercise of the Over-Allotment Option). Each Compensation Option shall be exercisable into one Compensation Unit consisting of one Common Share (a “Compensation Share”) and one Warrant (a “Compensation Warrant”) at the Offering Price for a period of 24 months following the Closing Date, subject to adjustment in certain customary events. Each Compensation Warrant will entitle the holder thereof to acquire one Common Share (each, a “Compensation Warrant Share”) at the Exercise Price for a period of twenty-four (24) months following the Closing Date, subject to acceleration on the same terms as the Warrants. This Prospectus qualifies the distribution of the Compensation Options to the Underwriters. See “Plan of Distribution”.
(3) After deducting the Underwriters’ Fee, but before deducting the expenses of the Offering, estimated to be $450,000 (excluding taxes and disbursements), which, together with the Underwriters’ Fee, will be paid out of the gross proceeds of the Offering.
(4) The Underwriters have been granted an over-allotment option exercisable in whole or in part, at the sole discretion of the Underwriters by giving notice to the Company at any time, and from time to time, on or before 5:00 p.m. (EDT) on the 30th day following the Closing Date, to purchase up to an additional 15% of the number of Offered Units sold under the Offering, being up to 4,350,000 Offered Units (the “Over-Allotment Units”) and/or up to
4,350,000 Unit Shares (“Over-Allotment Unit Shares”) and/or up to 4,350,000 Warrants (“Over-Allotment Warrants”), to cover the Underwriters’
over-allocation position, if any, and for market stabilization purposes (the “Over-Allotment Option”). The Over-Allotment Option may be exercised by the Underwriters to acquire: (a) Over-Allotment Units at the Offering Price; (b) Over-Allotment Unit Shares at a price of $0.67 per Over-Allotment Unit Share; (c) Over-Allotment Warrants at a price of $0.08 per Over-Allotment Warrant; or (d) any combination of Over-Allotment Units, Over-Allotment Unit Shares and Over-Allotment Warrants, so long as the aggregate number of Over-Allotment Unit Shares and Over-Allotment Warrants which may be issued under the Over-Allotment Option does not exceed 4,350,000 Over-Allotment Unit Shares and 4,350,000 Over-Allotment Warrants. If the Over- Allotment Option is exercised in full, the total “Price to the Public”, “Underwriters’ Fee” and “Net Proceeds to the Company” will be $25,012,500,
$1,500,750 and $23,511,750, respectively. This Prospectus qualifies the grant of the Over-Allotment Option and the distribution of the Over-Allotment Units issuable upon exercise of the Over-Allotment Option. A purchaser who acquires Over-Allotment Units, Over-Allotment Unit Shares or Over- Allotment Warrants forming part of the Underwriters’ over-allocation position acquires those Over-Allotment Units, Over-Allotment Unit Shares or Over- Allotment Warrants under this Prospectus, regardless of whether the over-allocation position is ultimately filled through the exercise of the Over- Allotment Option or secondary market purchases. See “Plan of Distribution”.
The following table sets out information relating to the Over-Allotment Option and the Compensation Options:
Underwriters’ Position
| Maximum Number of
Securities Available
|
Exercise Period
|
Exercise Price
|
| Up to 4,350,000 Over- Allotment Units, Over-
|
For a period of 30 days from
| $0.75 per Over-Allotment
Unit, $0.67 per Over-
|
Over-Allotment Option
| Allotment Unit Shares
| and including the Closing
| Allotment Unit Share and
|
| and/or Over-Allotment
| Date
| $0.08 per Over-Allotment
|
| Warrants
|
| Warrant
|
Compensation Options
| 2,001,000 Compensation
Options (including upon
|
24 months from the Closing
|
$0.75 per Compensation
|
| exercise of the Over-
| Date
| Option
|
| Allotment Option)
|
|
|
ii
Unless the context otherwise requires, when used herein, all references to “Offering” include the exercise of the Over-Allotment Option and all references to “Offered Units”, “Unit Shares”, “Warrants” and “Warrant Shares” include the securities underlying the exercise of the Over-Allotment Units.
The Underwriters, as principals, conditionally offer the Offered Units, subject to prior sale, if, as and when issued by the Company and accepted by the Underwriters in accordance with the conditions contained in the Underwriting Agreement referred to under “Plan of Distribution” and subject to the approval of certain legal matters on behalf of the Company by Gowling WLG (Canada) LLP and on behalf of the Underwriters by Borden Ladner Gervais LLP. An investment in the Offered Units involves a high degree of risk and should only be made by persons who can afford the total loss of their investment. Before purchasing the Offered Units, prospective purchasers should carefully review and evaluate the risk factors described under “Risk Factors” in this Prospectus and in the Annual Information Form (as defined herein), which can be found on the Company’s profile on SEDAR at www.sedar.com. Prospective purchasers are advised to consult their own tax advisors regarding the application of Canadian federal income tax laws to their particular circumstances, as well as any other provincial, foreign and other tax consequences of acquiring, holding or disposing of the Offered Units, Unit Shares, Warrants and/or Warrant Shares. See “Cautionary Note Regarding Forward- Looking Statements” and “Risk Factors”.
Prospective purchasers should rely only on the information contained or incorporated by reference in this Prospectus. The Company and the Underwriters have not authorized anyone to provide prospective purchasers with information different from that contained or incorporated by reference in this Prospectus. The Underwriters are offering to sell and seeking offers to buy the Offered Units only in jurisdictions where, and to persons to whom, offers and sales are lawfully permitted. Prospective purchasers should not assume that the information contained in this Prospectus is accurate as of any date other than the date on the cover page of this Prospectus.
Subscriptions for the Offered Units will be received subject to rejection or allotment, in whole or in part, and the Underwriters reserve the right to close the subscription books at any time without notice. Closing of the Offering (the “Closing”) is expected to take place on or about September 24, 2020 or such other date as the Underwriters and the Company may mutually agree (the “Closing Date”), acting reasonably, provided that the Offered Units are to be taken up by the Underwriters on or before the date that is not later than 42 days after the date of the receipt for the (final) short form prospectus relating to the Offering. See “Plan of Distribution”.
In connection with the Offering, and subject to applicable laws, the Underwriters may over-allot or effect transactions that are intended to stabilize or maintain the market price of the Unit Shares at levels other than that which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time. The Underwriters may offer the Offered Units at a lower price than stated above. See “Plan of Distribution”.
It is anticipated that the Offered Units will be delivered under the book-based system through CDS Clearing and Depository Services Inc. (“CDS”) or its nominee and deposited in electronic form. A purchaser of Offered Units will receive only a customer confirmation from the Underwriters or another registered dealer from or through which the Offered Units are purchased and who is a CDS depository service participant (a “Participant”). CDS will record the Participants who hold Unit Shares and Warrants comprising the Offered Units on behalf of owners who have purchased Offered Units in accordance with the book-based system. No certificates evidencing the Unit Shares or Warrants comprising the Offered Units will be issued to subscribers, except in certain limited circumstances, and registration will be made in the name of the nominee of CDS. Notwithstanding the foregoing, all Offered Units, Unit Shares and Warrants and any Warrant Shares, offered and sold in the United States or to or for the account or benefit of U.S. Persons who are institutional “accredited investors” as such term is defined in Rule 501(a)(1), (2), (3) or (7) of Regulation D promulgated under the U.S. Securities Act (the “U.S. Accredited Investors”), and who are not “qualified institutional buyers,” as such term is defined in Rule 144A under the U.S. Securities Act (“Qualified Institutional Buyers”, and together with the U.S. Accredited Investors, the “U.S. Purchasers”) will be issued in certificated, individually registered form. See “Plan of Distribution”.
A controlling shareholder of PI Financial Corp. is concurrently an influential shareholder of Bridging Finance Inc. (“Bridging”), which is a lender to certain subsidiaries of the Company (being Mid-American Growers, Inc. (“MAG”) and RWB Illinois, Inc. (“RWB Illinois”)) pursuant to the Amended Facility (as defined below) under which such subsidiaries are currently indebted and which the Company has guaranteed. Consequently, the Company may be considered to be a “connected issuer” (within the meaning of National
3
Instrument 33-105 – Underwriting Conflicts) of PI Financial Corp. under applicable Canadian securities legislation. See “Relationship Between the Company and Certain Underwriters”.
In this Prospectus, references to “RWB”, the “Company”, “we”, “us” and “our” refer to Red White & Bloom Brands Inc. and/or, as applicable, one or more of its subsidiaries. The Company’s head office and registered office is located at 789 West Pender Street, Suite 810, Vancouver, BC, V6C 1H2.
This Prospectus qualifies the distribution of securities of an entity that currently derives and intends to derive, directly, a substantial portion of its revenues from the cannabis industry in certain U.S. states, which industry is illegal under U.S. Federal law and enforcement of relevant laws is a significant risk. The Company is directly involved through certain subsidiaries and investees and expects to be directly involved through additional subsidiaries and proposed acquisition targets in the medical and adult-use cannabis industry in the States of Michigan, Illinois, California and Massachusetts which states have regulated such industries. Currently, the Company is directly engaged in, or pursuing operations regarding, the cultivation, possession, use, sale and distribution of medical and adult-use cannabis in such states. The Company, through certain subsidiaries and investees, has limited indirect operations in Florida and Oklahoma through sublicensing arrangements with arm’s length entities. The Company does not currently hold any cannabis licenses or have any pending cannabis license applications in either Florida or Oklahoma.
The United States federal government regulates drugs, in part, through the Controlled Substances Act (21 U.S.C. § 811) (the “Controlled Substances Act”), which places controlled substances, including cannabis, in a schedule. Cannabis is classified as a Schedule I drug. Under United States federal law, a Schedule I drug or substance has a high potential for abuse, no accepted medical use in the United States, and a lack of accepted safety for the use of the drug under medical supervision. The United States Food and Drug Administration has not approved marijuana as a safe and effective drug for any indication.
In the United States, marijuana is regulated at both the federal and state level, however these regulations are in direct conflict. State laws regulating cannabis are in direct conflict with the federal Controlled Substances Act, which makes cannabis use and possession federally illegal. Although certain states authorize medical or adult-use cannabis production and distribution by licensed or registered entities, under U.S. federal law, the possession, use, cultivation, and transfer of cannabis and any related drug paraphernalia is illegal, and any such acts are criminal acts under federal law. The Supremacy Clause of the United States Constitution establishes that the United States Constitution and federal laws made pursuant to it are paramount and, in case of conflict between federal and state law, the federal law shall apply. Third party service providers could suspend or withdraw services as a result of the Company operating in an industry that is illegal under United States federal law.
On January 4, 2018, then United States Attorney General Sessions issued a memorandum (the “Sessions Memo”) to all United States Attorneys which rescinded previous guidance from the U.S. Department of Justice specific to cannabis enforcement in the United States, including the Cole Memo (as defined herein). With the Cole Memo rescinded, and United States federal prosecutors having no further guidance relating to prosecution of cannabis-related violations of U.S. federal law, discretion on whether or not to prosecute such alleged violations has reverted to each respective U.S. Attorney to make such a determination. In the absence of such uniform federal guidance, as had been established by the Cole Memo, numerous United States Attorneys with state-legal marijuana programs within their jurisdictions have announced enforcement priorities for their respective offices. For instance, Andrew Lelling, United States Attorney for the District of Massachusetts, stated that while his office would not immunize any businesses from federal prosecution, he anticipated focusing the office’s marijuana enforcement efforts on: (1) overproduction; (2) targeted sales to minors; and (3) organized crime and interstate transportation of drug proceeds. Other United States Attorneys provided less assurance, promising to enforce federal law, including the Controlled Substances Act in appropriate circumstances. United States Attorney General Sessions resigned on November 7, 2018. He was replaced by William Barr on February 14, 2019. It is unclear what specific impact this development will have on U.S. federal government enforcement policy as the Department of Justice under Mr. Barr has not taken a formal position on federal enforcement of laws relating to cannabis. However, during his confirmation, and in response to written inquiries by U.S. Senators, Mr. Barr stated that “[he does] not
4
intend to go after parties who have complied with state law in reliance on the Cole Memorandum.” Mr. Barr has also stated that, while his preference would be to have a uniform federal rule addressing cannabis; absent such a uniform position, his preference would be to permit the existing federal approach of allowing individual states or territories to determine cannabis policy and to trust the judgment of U.S. prosecutors on how to enforce U.S. federal law. If the Department of Justice policy under Attorney General Barr was to prosecute cannabis-related business, including but not limited to any investors, financiers, employees, officers and managers, and United States Attorneys followed such Department of Justice policies through pursuing prosecutions, then the Company could face (i) seizure of its cash and other assets used to support or derived from its cannabis operations; (ii) the arrest of its employees, directors, officers and managers; and (iii) the barring of its employees, directors, officers, managers and investors who are not United States citizens from entry into the United States. There is no guarantee that state laws legalizing and regulating the sale and use of cannabis will not be repealed or overturned, or that local governmental authorities will not limit the applicability of state laws within their respective jurisdictions. Unless and until the United States Congress amends U.S. federal law with respect to cannabis (and as to the timing or scope of any such potential amendments there can be no assurance), there is a risk that federal authorities may enforce current U.S. federal law. If the United States federal government begins to enforce United States federal laws relating to cannabis in states where the sale and use of cannabis is currently legal under state law, or if existing applicable state laws are repealed or curtailed, the Company’s business, results of operations, financial condition and prospects would be materially adversely affected.
Although the Cole Memo has been rescinded, one legislative safeguard for the medical marijuana industry remains in place: Congress has passed a so-called “rider” provision in the FY 2015, 2016, 2017 and 2018 Consolidated Appropriations Acts to prevent the federal government from using congressionally appropriated funds to enforce federal marijuana laws against regulated medical marijuana actors operating in compliance with state and local law. The rider is known as the “Rohrabacher-Farr” Amendment after its original lead sponsors (it is also sometimes referred to as the “Rohrabacher-Blumenauer” or “Joyce-Leahy” Amendment, but it is referred to in this Prospectus as “Rohrabacher-Farr”). Most recently, the Rohrabacher-Farr Amendment was included in the Consolidated Appropriations Act of 2019, which was signed by President Donald Trump on February
14, 2019 and funds the departments of the federal government through the fiscal year ending September
30, 2019. In signing the Act, President Trump issued a signing statement noting that the Act “provides that the Department of Justice may not use any funds to prevent implementation of medical marijuana laws by various States and territories,” and further stating “I will treat this provision consistent with the President’s constitutional responsibility to faithfully execute the laws of the United States.” While the signing statement can fairly be read to mean that the executive branch intends to enforce the Controlled Substances Act and other federal laws prohibiting the sale and possession of medical marijuana, the President did issue a similar signing statement in 2017 and no major federal enforcement actions followed. On December 20, 2019, President Trump signed the 2020 Fiscal Year Appropriations Bill which included the Rohrabacher-Farr Amendment, which prohibits the funding of federal prosecutions with respect to medical cannabis activities that are legal under state law, extending its application until September 30, 2020. There can be no assurances that the Rohrabacher/Blumenauer Amendment will be included in future appropriations bills. See “Regulatory Overview – U.S. Federal Regulatory Landscape”.
There is no guarantee that state laws legalizing and regulating the sale and use of cannabis will not be repealed or overturned, or that local governmental authorities will not limit the applicability of state laws within their respective jurisdictions. Unless and until the United States Congress amends the Controlled Substances Act with respect to marijuana (and as to the timing or scope of any such potential amendments there can be no assurance), there is a risk that federal authorities may enforce current U.S. federal law. If the United States federal government begins to enforce United States federal laws relating to cannabis in states where the sale and use of cannabis is currently legal, or if existing applicable state laws are repealed or curtailed, the Company’s business, results of operations, financial condition and prospects would be materially adversely affected.
Marijuana remains a Schedule I controlled substance at the federal level, and neither the Cole Memo nor its rescission nor the continued passage of the Rohrabacher-Farr Amendment has altered that fact. The federal government of the United States has always reserved the right to enforce federal law in regard
5
to the sale and disbursement of medical or adult-use marijuana, even if state law sanctions such sale and disbursement. If the United States federal government begins to enforce United States federal laws relating to cannabis in states where the sale and use of cannabis is currently legal, or if existing applicable state laws are repealed or curtailed, the Company’s business, results of operations, financial condition and prospects would be materially adversely affected.
Additionally, under United States federal law, it may potentially be a violation of federal anti-money laundering statutes for financial institutions to take any proceeds from the sale of any Schedule I controlled substance. Due to the Controlled Substances Act categorization of marijuana as a Schedule I drug, federal law makes it illegal for financial institutions that depend on the Federal Reserve’s money transfer system to take any proceeds from marijuana sales as deposits. Banks and other financial institutions could be prosecuted and possibly convicted of money laundering for providing services to cannabis businesses under the United States Currency and Foreign Transactions Reporting Act of 1970 (the “Bank Secrecy Act”) as amended by Title III of the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001. Therefore, under the Bank Secrecy Act, banks or other financial institutions that provide a cannabis business with a checking account, debit or credit card, small business loan, or any other service could be charged with money laundering or conspiracy. Despite the absence of express banking protections under U.S. federal law, the FinCEN Guidance (as described herein), which was adopted by the U.S. Department of the Treasury in 2014 and remains in place presently, advised prosecutors not to focus their enforcement efforts on banks and other financial institutions that serve marijuana-related businesses so long as that business is legal in their state and none of the federal enforcement priorities referenced in the Cole Memo are being violated.
The Company’s objective is to capitalize on the opportunities presented as a result of the changing regulatory environment governing the cannabis industry in the United States. Accordingly, there are a number of significant risks associated with the business of the Company. Unless and until the U.S. Congress amends the Controlled Substances Act with respect to adult-use cannabis (and as to the timing or scope of any such potential amendments there can be no assurance), there is a risk that federal authorities may enforce current federal law, and the business of the Company may be deemed to be producing, cultivating, extracting, or dispensing cannabis or aiding or abetting or otherwise engaging in a conspiracy to commit such acts in violation of federal law in the United States.
In light of the political and regulatory uncertainty surrounding the treatment of United States cannabis related activities, on February 8, 2018, the Canadian Securities Administrators published CSA Staff Notice 51-352 – (Revised) Issuers with U.S. Marijuana-Related Activities (the “Staff Notice 51-352”) setting out the Canadian Securities Administrator’s disclosure expectations for specific risks facing issuers with cannabis related activities in the United States. Staff Notice 51-352 includes additional disclosure expectations that apply to all issuers with United States cannabis-related activities, including those with direct and indirect involvement in the cultivation and distribution of cannabis, as well as issuers that provide goods and services to third parties involved in the United States cannabis industry. The Company is directly involved through certain subsidiaries and investees in the cultivation and distribution of cannabis in the United States for purposes of Staff Notice 51-352.
For these reasons, the Company’s involvement in the U.S. cannabis market may subject the Company to heightened scrutiny by regulators, stock exchanges, clearing agencies and other U.S. and Canadian authorities. There can be no assurances that this heightened scrutiny will not in turn lead to the imposition of certain restrictions on the Company’s ability to operate in the United States or any other jurisdiction. There are a number of risks associated with the business of the Company. See the sections entitled “Regulatory Overview” and “Risk Factors” in this Prospectus, and the sections entitled “General Development of the Business – Trends, Commitments, Events or Uncertainties” and “Risk Factors” in the Listing Statement (as defined herein).
6
TABLE OF CONTENTS
GENERAL MATTERS.............................................................................................................................................. 1
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS......................................................... 1
MARKET AND INDUSTRY DATA ........................................................................................................................... 3
MARKETING MATERIALS ...................................................................................................................................... 3
ELIGIBILITY FOR INVESTMENT ............................................................................................................................ 3
DOCUMENTS INCORPORATED BY REFERENCE .............................................................................................. 4
THE COMPANY....................................................................................................................................................... 6
RECENT DEVELOPMENTS ................................................................................................................................... 9
REGULATORY OVERVIEW.................................................................................................................................. 10
CONSOLIDATED CAPITALIZATION .................................................................................................................... 40
USE OF PROCEEDS ............................................................................................................................................ 42
MARKET FOR SECURITIES................................................................................................................................. 44
DESCRIPTION OF SECURITIES BEING DISTRIBUTED .................................................................................... 46
PLAN OF DISTRIBUTION ..................................................................................................................................... 49
RELATIONSHIP BETWEEN THE COMPANY AND CERTAIN UNDERWRITERS .............................................. 52
RISK FACTORS .................................................................................................................................................... 52
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS................................................................. 63
STATUTORY RIGHTS OF WITHDRAWAL AND RESCISSION........................................................................... 67
INTEREST OF EXPERTS ..................................................................................................................................... 67
AUDITORS, TRANSFER AGENT AND REGISTRAR........................................................................................... 67
CERTIFICATE OF THE COMPANY……………………………………………………………………………………. C-1
CERTIFICATE OF THE UNDERWRITERS……………………………………………………………………………. C-2
GENERAL MATTERS
Purchasers should rely only on the information contained in or incorporated by reference into this Prospectus and are not entitled to rely on parts of the information contained in this Prospectus to the exclusion of others. The Company and the Underwriters have not authorized anyone to provide purchasers with additional or different information. Information contained on the Company’s website shall not be deemed to be a part of this Prospectus or incorporated by reference herein and may not be relied upon by prospective purchasers for the purpose of determining whether to invest in the securities qualified for distribution under this Prospectus. The Offered Units are not being offered or sold in any jurisdiction where the offer or sale is not permitted. Prospective purchasers should assume that the information appearing or incorporated by reference in this Prospectus is accurate only as at the respective dates thereof, regardless of the time of delivery of the Prospectus or of any sale of the Offered Units. The Company’s business, financial condition, results of operations and prospects may have changed since that date. The Company does not undertake to update the information contained or incorporated by reference herein except as required by applicable Canadian securities laws.
This Prospectus shall not be used for any purpose other than in connection with the Offering.
Except as otherwise indicated, references to “Canadian dollars” or “$” are to the currency of Canada.
This Prospectus, including the documents incorporated by reference herein, contains company names, product names, trade names, trademarks and service marks of the Company and other organizations, all of which are the property of their respective owners.
The documents incorporated or deemed to be incorporated by reference herein contain meaningful and material information relating to the Company and prospective purchasers should review all information contained in this Prospectus and the documents incorporated or deemed to be incorporated by reference herein.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Prospectus, including the documents incorporated by reference herein, contains forward-looking statements or information (collectively “forward-looking statements”) which are based upon the Company’s current internal expectations, estimates, projections, assumptions and beliefs. In some cases, these forward-looking statements can be identified by words or phrases such as “may”, “believes”, “expects”, “will”, “intends”, “projects”, “anticipates”, “estimates”, “continues”, “plans”, “aim”, “seek” or the negative of these terms, or other similar expressions intended to identify forward-looking statements. The Company has based these forward-looking statements on current expectations and projections about future events and financial trends that they believe may affect the Company’s financial condition, results of operations, business strategy and financial needs, as the case may be.
Forward-looking statements relating to the Company include, among other things, statements relating to:
* the completion of the Offering and the receipt of all regulatory and CSE approvals in connection therewith;
* the listing on the CSE of the Unit Shares, the Warrants and Warrant Shares (including the Compensation Shares, the Compensation Warrants and the Compensation Warrant Shares)
* the Company’s use of proceeds and business objectives and milestones and the anticipated timing of execution, see “Use of Proceeds”;
* the performance of the Company’s business and operations;
* the successful completion of the Company’s previously announced transactions, including, but not limited to, the Company’s proposed acquisition of Platinum Vape, LLC (“Platinum Vape”), the Company’s proposed acquisition of PharmaCo Inc. (“PharmaCo”), the Company’s exclusive partnership for the distribution and commercialization of Avicanna Inc. (“Avicanna”) products, the Company’s growing and sales agreement with 39 Industries, LLC (operating as Critical 39) (“Critical 39”);
* the intention to expand the business, operations and potential activities of the Company;
current legislation in the United States and various states thereof pertaining to the production, distribution, sale and use of medical and recreational cannabis;
* future prices and demand for cannabis in the United States and the supply of cannabis and the production of products derived therefrom;
* development of projects in which the Company invests being on time and on budget;
* the accuracy and veracity of information and projections sourced from third parties respecting, among other things, future industry conditions and demand for cannabis;
* the competitive conditions of the cannabis industry;
* the competitive and business strategies of the Company;
* the Company’s anticipated operating cash requirements and future financing needs;
* the anticipated future gross revenues and profit margins of the Company’s operations;
* the Company’s expectations regarding its revenue, expenses and operations;
* impacts of potential litigation;
* the Company’s intention to build brands and develop cannabis products targeted to specific segments of the market;
* the ongoing and proposed expansion of the Company’s facilities, products or services, including associated costs and any applicable licencing;
* the current political, legal and regulatory landscape surrounding medical and recreational cannabis and expected developments in any jurisdiction in which the Company operates or may operate;
* the receipt of any regulatory and stock exchange approvals required at any given time;
* the applicable laws, regulations and any amendments thereof;
* medical benefits, viability, safety, efficacy and dosing of cannabis;
* the expected growth in the number of patients;
* the expected number of grams of medical cannabis used by each patient;
* expectations with respect to the advancement and adoption of new product lines and ingredients;
* the acceptance by customers and the marketplace of new products and solutions;
* the ability to attract new customers and develop and maintain existing customers;
* expectations with respect to future production costs and capacity;
* expectations and anticipated impact of the COVID-19 pandemic;
* expectations with respect to the receipt, renewal, amendment and/or extension of the Company’s permits and licences;
* the ability to protect, maintain and enforce the Company’s intellectual property rights;
* the ability to successfully leverage current and future strategic partnerships and alliances;
* the ability to attract and retain personnel;
* anticipated labour and materials costs;
* the Company’s competitive condition and expectations regarding competition, including pricing and demand expectations and the regulatory environment in which the Company operates; and
* anticipated trends and challenges in the Company’s business and the markets and jurisdictions in which the Company operates or may operate.
Forward-looking statements are based on certain key assumptions and analyses made by the Company in light of its experience and perception of historical trends, current conditions and expected future developments and other factors the Company believes are appropriate and are subject to risks and uncertainties and include assumptions made by the Company about its business, the economy and the cannabis industry in general, particularly in light of the impact of the COVID-19 virus (“COVID-19”). Although management believes that the assumptions underlying these statements are reasonable, they may prove to be incorrect. Given these risks, uncertainties and assumptions, shareholders and prospective purchasers of the Company’s securities should not place undue reliance on these forward-looking statements. The above list of forward-looking statements is not exhaustive and whether actual results, performance or achievements will conform to the Company’s expectations and predictions is subject to a number of known and unknown risks, uncertainties, assumptions and other factors.
Further, any forward-looking statement speaks only as of the date on which such statement is made, and, except as required by applicable law, the Company undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date on which such statement is made or to reflect the occurrence of unanticipated events.
Certain of the forward-looking statements contained herein concerning cannabis, the general expectations of the Company related thereto, and the Company’s business and operations are based on estimates prepared by the Company using data from publicly available governmental sources, as well as from market research and industry
-2-
analysis and on assumptions based on data and knowledge of this industry which the Company believes to be reasonable. However, although generally indicative of relative market positions, market shares and performance characteristics, such data is inherently imprecise. While the Company is not aware of any misstatement regarding any industry or government data presented herein, the current cannabis industry involves risks and uncertainties that are subject to change based on various factors. It is not possible for management to predict all such factors and to assess in advance the impact of each such factor on the Company’s business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward- looking statement. Readers are cautioned that actual future results may differ materially from management’s current expectations and the forward-looking statements contained in this Prospectus and the documents incorporated by reference herein are expressly qualified in their entirety by this cautionary statement. For a description of material factors that could cause the Company’s actual results to differ materially from the forward-looking statements in this Prospectus, please see “Risk Factors” in this Prospectus and in the Company’s Annual Information Form for the period ended July 31, 2019, filed under the Company’s profile on SEDAR and available at www.sedar.com.
MARKET AND INDUSTRY DATA
Market and industry data contained and incorporated by reference in this Prospectus concerning economic and industry trends is based upon good faith estimates of our management or derived from information provided by industry sources. The Company believes that such market and industry data is accurate and that the sources from which it has been obtained are reliable. However, we cannot guarantee the accuracy of such information and we have not independently verified the assumptions upon which projections of future trends are based.
MARKETING MATERIALS
Any “template version” of “marketing materials” (as such terms are defined in National Instrument 41-101 – General Prospectus Requirements) will be incorporated by reference into the final short form prospectus. However, any such template version of marketing materials will not form part of the final short form prospectus to the extent that the contents of the template version of marketing materials are modified or superseded by a statement contained in the final short form prospectus. Any template version of marketing materials filed after the date of this Prospectus and before the termination of the distribution under the Offering (including any amendments to, or an amended version of, the Marketing Materials (as defined herein)) is deemed to be incorporated in this Prospectus.
ELIGIBILITY FOR INVESTMENT
In the opinion of Gowling WLG (Canada) LLP, counsel to the Company, and Borden Ladner Gervais LLP, counsel to the Underwriters, based on the current provisions of the Income Tax Act (Canada) and the regulations thereunder (collectively, the “Tax Act”) as of the date hereof, the Unit Shares, the Warrants and the Warrant Shares, if issued on the date hereof, would be “qualified investments” under the Tax Act for trusts governed by registered retirement savings plans, registered retirement income funds, registered education savings plans, registered disability savings plans and tax-free savings accounts, each as defined in the Tax Act (collectively “Registered Plans”) and trusts governed by deferred profit sharing plans (“DPSPs”), provided that:
(i) in the case of Unit Shares and Warrant Shares, the Common Shares are listed on a “designated stock exchange” as defined in the Tax Act (which currently includes the CSE) or the Company qualifies as a “public corporation” (as defined in the Tax Act); and
(ii) in the case of Warrants, the Warrants, once listed on a “designated stock exchange” as defined in the Tax Act (which currently includes the CSE), or the Warrant Shares are qualified investments as described in (i) above and neither the Company, nor any person with whom the Company does not deal at arm’s length, is an annuitant, a beneficiary, an employer or subscriber under or a holder of such Registered Plan or DPSP.
Notwithstanding the foregoing, holders, annuitants or subscribers of Registered Plans (each a “Controlling Individual”) will be subject to a penalty tax in respect of the Unit Shares, Warrants and Warrant Shares held in a trust governed by a Registered Plan if such Unit Shares, Warrants or Warrant Shares, as the case may be, are a
-3-
“prohibited investment” under the Tax Act for the particular Registered Plan. Unit Shares, Warrants or Warrant Shares will generally not be a “prohibited investment” for a Registered Plan unless the Controlling Individual of the Registered Plan (i) does not deal at arm’s length with the Company for purposes of the Tax Act; or (ii) has a “significant interest”, as defined in the Tax Act, in the Company. In addition, the Unit Shares and Warrant Shares will not be a “prohibited investment” if such securities are “excluded property” (as defined in the Tax Act for purposes of the prohibited investment rules) for trusts governed by a Registered Plan.
Persons who intend to hold Unit Shares, Warrants or Warrant Shares in a Registered Plan or DPSP, should consult their own tax advisors in regard to the application of these rules in their particular circumstances.
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this Prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the documents incorporated herein by reference may be obtained on request without charge from the Corporate Secretary of the Company at 789 West Pender Street, Suite 810, Vancouver, BC, V6C 1H2, Telephone 604-687-2038, and are also available electronically on SEDAR at www.sedar.com.
The following documents of the Company filed with the securities commissions or similar authorities in Canada are incorporated by reference in this Prospectus:
1. the Company’s annual information form dated August 7, 2020 (the “Annual Information Form”) in respect of the fiscal year ended July 31, 2019 (excluding any reference in the Annual Information Form to the Company as a passive foreign investment company (“PFIC”). See “Risk Factors – Risks Related to the Business – Passive Foreign Investment Company Status”;
2. the Company’s CSE Form 2A listing statement dated June 1, 2020 respecting the business combination transaction (the “Business Combination Transaction”) involving the Company (formerly Tidal Royalty Corp. (“Tidal”)) and MichiCann Medical Inc. (“MichiCann”) (excluding Tidal’s interim financial statements and management’s discussion and analysis thereon) (the “Listing Statement”);
3. the Company’s audited consolidated financial statements and the notes thereto as at and for the years ended July 31, 2019 and 2018, together with the auditor’s report thereon (the “Annual Financial Statements”);
4. the Company’s management’s discussion and analysis for the years ended July 31, 2019 and 2018 (the
“Annual MD&A”);
5. the Company’s statement of executive compensation for the financial years ended July 31, 2019 and 2018;
6. the Company’s audited consolidated financial statements in respect of MichiCann and the notes thereto as at and for the years ended December 31, 2019 and 2018, together with the auditor’s report thereon;
7. the Company’s management’s discussion and analysis of financial conditions and operations in respect of
MichiCann for the years ended December 31, 2019 and 2018;
8. the Company’s unaudited condensed interim consolidated financial statements and the notes thereto as at and for the three and six months ended January 31, 2020 and 2019;
9. the Company’s management’s discussion and analysis for the six month period ended January 31, 2020 and 2019;
10. the Company’s reviewed amended and restated unaudited condensed interim consolidated financial statements and the notes thereto as at and for the three and six months ended June 30, 2020 and 2019 (the “Interim Financial Statements”);
11. the Company’s amended and restated management’s discussion and analysis for the three and six months ended June 30, 2020;
12. the Company’s management information circular dated August 5, 2020 respecting an annual and special meeting of shareholders of the Company;
-4-
13. the material change report dated August 24, 2020 respecting: (i) the entering into by the Company on July 24, 2020 of a growing and sales agreement with Critical 39 (the “Critical 39 Agreement”); (ii) the entering into by the Company on August 11, 2020 of a distribution agreement with Avicanna for the exclusive distribution of Avicanna’s advanced and clinically backed cannabidiol (“CBD”) based cosmetic and topical products Pura H&W™ in the United States and certain other markets (the “Avicanna Distribution Agreement”); (iii) the Company’s providing of notice to PharmaCo shareholders (the “PharmaCo Shareholders”) on July 24, 2020 of its intent to exercise its right to acquire 100% of the issued and outstanding shares of PharmaCo pursuant to the put/call option agreement dated January 4, 2019 between MichiCann, PharmaCo and PharmaCo Shareholders (the “PharmaCo Put/Call Agreement”); (iv) the entering into by the Company on July 21, 2020 of a binding letter of intent to acquire 100% of the issued and outstanding shares of Platinum Vape; and (v) the appointment of CNBC Market Analyst Steven Grasso as Business Advisor.
14. the material change report dated July 7, 2020 respecting a debt settlement subscription agreement with an arm-length investor entered into on June 30, 2020 to settle advances made by the investor to PharmaCo;
15. the material change reports dated June 8, 2020 and June 11, 2020 respecting the Company’s acquisition on June 10, 2020 of 1251881 B.C. Ltd. (“Newco”), being the entity holding the licensing rights for the branding of High Times® (“High Times”) dispensaries and High Times cannabis-based CBD and THC products in the states of Michigan, Illinois and Florida and branding of High Times hemp-derived CBD products nationally in the United States carrying the Culture® brand pursuant to a retail license agreement and a product license agreement with HT (as defined below), which transactions were completed by way of a three-cornered amalgamation under the Business Corporations Act (British Columbia), whereby 1252034 B.C. Ltd., a wholly-owned British Columbia subsidiary of RWB, amalgamated with 1251881 B.C. Ltd. to form RWB Licensing Inc. (“RWB Licensing”) in exchange for the issuance to 1252240 B.C. Ltd. (the “Seller”), a wholly-owned subsidiary of HT Retail Licensing, LLC (“HT”) of: (i) 13,500,000 Common Shares issued at a deemed price of $1.50 per Common Share; and (ii) a special warrant of the Company that is exercisable into 4,500,000 additional Common Shares if the volume weighted average price of the Common Shares on the CSE, for the first 180 days following June 10, 2020 is below $1.50, all pursuant to an acquisition agreement between the Company, HT, the Seller and Newco dated June 4, 2020 (the “RWB Licensing Acquisition”);
16. the material change report dated June 8, 2020 respecting the resumption of trading of the Company’s shares on the CSE;
17. the material change report dated April 29, 2020 respecting the completion of the Company’s Business Combination Transaction with MichiCann on April 24, 2020 whereby (i) the Company changed its name from “Tidal Royalty Corp.” to “Red White & Bloom Brands Inc.” and completed a 16:1 share consolidation including common shares, series I convertible preferred shares (the “Series I Preferred Shares”), options and warrants; (ii) the Company fixed the number of directors at five and appointed Brad Rogers, Johannes (Theo) van der Linde, Brendan Purdy, Michael Marchese and William Dawson; (iii) appointed Brad Rogers as Chief Executive Officer and Johannes (Theo) van der Linde as Chief Financial Officer; (iv) the Company issued Common Shares, series II convertible preferred shares (the “Series II Preferred Shares”), warrants and options to former holders of MichiCann common shares, warrants and options; (v) certain shareholders entered into voluntary escrow agreements; and (vi) the Company agreed to guarantee certain obligations of PharmaCo, MAG and RWB Illinois pursuant to an amended and restated credit agreement with Bridging dated January 10, 2020;
18. the material change report dated March 13, 2020 respecting the entering into of an amended and restated business combination agreement with MichiCann in respect of the Business Combination Transaction;
19. the notice of change in corporate structure dated May 14, 2020 whereby, effective as of April 24, 2020, the Company changed its year end to December 31, 2020; and
20. the template version of the term sheet for the Offering dated August 21, 2020 (the “Marketing Materials”);
21. the material change report dated August 28, 2020 respecting the announcement of the Offering;
22. the material change report dated September 8, 2020 respecting the definitive agreement with Platinum Vape (the “Platinum Vape Definitive Agreement”); and
-5-
23. the material change report dated September 15, 2020 respecting (i) the issuance of a $10 million convertible debenture to 1260356 Ontario Limited on September 11, 2020 (the “Convertible Debenture”) of which a portion of the proceeds was used to make the initial payment under the Platinum Vape Definitive Agreement; and (ii) the completion of the Platinum Vape acquisition on September 14, 2020 whereby RWB Platinum Vape Inc., a wholly-owned California subsidiary of RWB (“RWB Platinum Vape”), acquired all of the issued and outstanding equity interests of Platinum Vape.
Any documents of the type referred to above or similar material and any documents required to be incorporated by reference herein pursuant to National Instrument 44-101 – Short Form Prospectus Distributions, including any annual information form, all material change reports (excluding confidential reports, if any), all annual and interim financial statements and management’s discussion and analysis relating thereto, or information circular or amendments thereto that the Company files with any securities commission or similar regulatory authority in Canada after the date of this Prospectus and prior to the termination of this Offering will be deemed to be incorporated by reference in this Prospectus and will automatically update and supersede information contained or incorporated by reference in this Prospectus.
Any statement contained in this Prospectus or a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for purposes of this Prospectus, to the extent that a statement contained herein or in any other subsequently filed document that also is or is deemed to be incorporated by reference herein modifies, replaces or supersedes such statement. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made. Any statement so modified or superseded shall not constitute a part of this Prospectus, except as so modified or superseded.
THE COMPANY Summary of the Business
RWB focuses on investing in and financing businesses that pertain in any way to cannabis and which are carried out in compliance with applicable United States state laws (the “U.S. legal cannabis industry”). The Company actively seeks financing arrangements involving royalties, debt and other forms of investments and acquisitions in private and public companies in the U.S. legal cannabis industry. It is anticipated that the Company will predominately focus its investments, with the strength of its world‐class team, on major markets in the United States, including Michigan, Illinois, California, and Massachusetts with respect to cannabis and the entire United States for legal hemp CBD based products. The Company, through certain subsidiaries and investees, currently has limited indirect involvement in the U.S. legal cannabis industry in Florida and Oklahoma in the form of sublicensing arrangements with arm’s length entities.
The Company’s business objective is to provide capital solutions to companies in the U.S. legal cannabis industry with large-scale potential and a highly-skilled and experienced management team across multiple industry verticals, including cultivation, processing and distribution. The Company is actively pursuing opportunities to provide expansion capital to licensed, qualified operators across multiple industry verticals including cultivation, processing and distribution.
The nature and timing of the Company’s investments will depend, in part, on available capital at any particular time and the investment opportunities identified and available to the Company. As the board of directors of the Company (the “Board of Directors”) grows, the Company expects that it will establish a formal investment committee to evaluate future opportunities. Until such time as a formal committee is created, the Board of Directors will continue to carry out all functions in accordance with the Company’s investment policy. The Company expects its investment activities will be primarily focused on enterprises located in the United States, although investments may extend globally (including the purchase of securities listed on foreign stock exchanges). The Company expects to invest solely in the cannabis sector. The Company believes that any risk of limited diversification may be mitigated by
-6-
closely monitoring its investments. The actual composition of the Company’s investment portfolio will vary over time depending on its assessment of a number of factors, including the performance of United States cannabis markets and credit risk.
The Company’s current material subsidiaries and investees are MichiCann, PharmaCo, RWB Illinois, MAG, RWB Platinum Vape, Vista Prime Management, LLC, GC Ventures 2, LLC, Vista Prime 3, Inc., PV CBD LLC, Vista Prime 2, Inc., RWB Licensing, RLTY USA Corp., RLTY Development MA 1 LLC, RLTY Development Springfield LLC, RLTY Development Orange LLC, RLTY Beverage LLC and VLF Holdings LLC d/b/a Diem (“Diem”), the financing transactions in respect of each are described herein and in the Listing Statement.
For a detailed description of the business of the Company, prospective purchasers should refer to the Company’s Annual Information Form incorporated by reference into this Prospectus and available on the Company’s SEDAR profile at www.sedar.com.
Inter-Corporate Relationships
The following chart illustrates the Company’s material subsidiaries, the percentage of voting securities of each that are held by RWB either directly or indirectly, and their respective jurisdiction of incorporation, continuance, formation or organization:
Subsidiary Name
| Ownership by
RWB
| Jurisdiction of Incorporation,
Continuance, Formation or
Organization
|
RLTY USA Corp.
| 100%
| Delaware
|
RLTY Beverage 1 LLC
| 100%
| Delaware
|
RLTY Development MA 1 LLC
| 100%
| Delaware
|
RLTY Development Springfield LLC
| 100%
| Massachusetts
|
RLTY Development Orange LLC
| 100%
| Massachusetts
|
Michicann Medical Inc.
| 100%
| Ontario
|
Mid-American Growers, Inc.
| 100%
| Delaware
|
RWB Illinois, Inc.
| 100%
| Delaware
|
RWB Licensing Inc.
| 100%
| British Columbia
|
RWB Platinum Vape Inc.
| 100%
| California
|
Vista Prime Management, LLC
| 100%
| California
|
GC Ventures 2, LLC
| 100%
| Michigan
|
Vista Prime 3, Inc.
| 100%
| California
|
PV CBD LLC
| 100%
| California
|
Vista Prime 2, Inc.
| 100%
| California
|
-7-
Investees
The following table describes the Company’s material investees. Harborside Inc. and Lighthouse Strategies LLC were specifically excluded from the table as non-material investees.
PharmaCo
|
Incorporation date
| March 11, 2016
|
RWB ownership
| RWB holds a put/call option to acquire PharmaCo in exchange for 37,000,000 Common Shares and 37,000,000 Series II Preferred Shares pursuant to the PharmaCo Put/Call Agreement. As at June 30, 2020, the call option was determined to have a fair value of $19,828,224.
As at June 30, 2020, RWB has advanced an aggregate of $90,262,929 to PharmaCo through a promissory note and a senior secured convertible debenture, as further described in the Listing Statement.
|
interest and investment
amount
|
|
Management and
| James Skrinner, President and Director
Fernando Di Carlo, Vice-President and Director
PharmaCo operates at arm’s length to RWB, and as such RWB’s knowledge of the management and directors of PharmaCo is limited to information that is publicly available.
RWB does not have any compensation or employment arrangements with any members of management or the board of directors of PharmaCo.
|
directors, including
compensation and employment arrangements
|
|
|
|
|
Related party
transactions between
RWB and the investee
| None.
|
Exit strategy
| RWB has applied for regulatory approval to acquire PharmaCo and is currently awaiting a decision from Michigan regulatory authorities. If approved, RWB will assume the operation of PharmaCo’s business. In the event regulatory approval is not granted, RWB will evaluate options that include, among other things, terminating the relationship.
|
Management fee
| No current management fee arrangements. A previous management fee arrangement existed between PharmaCo and MichiCann whereby PharmaCo would pay US$100,000 per month for certain administrative, non-operational support services. PharmaCo retained outside service providers for these administrative services subsequent to Q1 2020.
|
arrangements
|
Diem
|
Incorporation date
| February 14, 2014
|
RWB ownership
| RWB executed a definitive agreement with Diem on August 31, 2018, as amended on October 15, 2018 and December 26, 2018 (the “Diem Agreement”), to finance the expansion of TDMA LLC, a Massachusetts subsidiary of Diem (“TDMA”) into Massachusetts. Pursuant to the Diem Agreement, the Company will provide Diem with up to US$12.5 million over three years to develop and operate a large-scale cultivation and processing facility and up to four dispensaries in Massachusetts (the “Diem Financing”).
The terms of the Diem Financing were re-negotiated and RWB began the process of exiting the investment pursuant to the terms of the Diem Agreement. This culminated in RWB entering into a definitive Membership Interest Purchase Agreement on September 26, 2019 (the “TDMA MIPA”) with TDMA to acquire all of the issued and
|
interest and investment
amount
|
|
-8-
| outstanding equity in TDMA Orange, LLC, a Diem subsidiary. Pursuant to the terms of the TDMA MIPA, RWB will acquire a 100% ownership interest in two cultivation licenses and a processing license in Orange, Massachusetts conditional upon approval of a change of control application. As consideration for the TDMA MIPA, RWB will forgive certain promissory notes including accrued interest, cross collateralization and general security arrangement. A change of control application for the TDMA Orange licenses was submitted in September 2020 and it is anticipated that it will take 5 to 8 months to be processed by the Cannabis Control Commission of the Commonwealth of the State of Massachusetts.
|
Management and
Directors, including
compensation and
employment
arrangements
| Chris Mitchem, Chief Executive Officer
Diem operates at arm’s length to RWB, and as such RWB’s knowledge of themanagement and directors of Diem is limited to information that is publicly available.
RWB does not have any compensation or employment arrangements with any members of management or the board of directors of Diem.
|
Related party
transactions between
RWB and the investee
| None.
|
Exit strategy
| Upon receipt of the licenses currently awaiting regulatory approval from state authorities, RWB will determine whether to pursue a business in Massachusetts or to liquidate the licensed entities.
|
Management fee
| None.
|
arrangements
|
RECENT DEVELOPMENTS
The following are material recent developments of the Company since the filing of the Annual Information Form. PharmaCo continues to produce an aggregate of 2,500,000 grams of medical cannabis per year on an annual
basis from two facilities in Michigan. PharmaCo’s planned 85,000 square foot facility capable of producing
10,000,000 grams of medical cannabis per year is still under development and not yet operational. PharmaCo has been purchasing wholesale flower under offtake agreements to fulfill its excess supply needs as construction of its new facility continues. PharmaCo intends to have at least 25 operating provisioning centers prior to the end of the
2020 calendar year, subject to financing and applicable regulatory approvals.
On August 11, 2020, RWB entered into the Avicanna Distribution Agreement for the exclusive distribution of Avicanna’s advanced and clinically backed Pura H&W™ CBD-based cosmetic and topical products by RWB in the United States and certain other markets.
Under the Avicanna Distribution Agreement, which has an initial five-year term, RWB will exclusively distribute the Pura H&W™ brand and certain other white label brands at RWB’s direction. RWB will pay Avicanna an upfront cash licensing fee in the amount of $250,000, along with minimum purchase requirements for the rights to be the exclusive distributor of Avicanna’s Pura H&W™ branded cosmetics products in the United States. Under the Avicanna Distribution Agreement, RWB also has the right to purchase Avicanna’s cosmetics products for distribution into the United States and certain other territories under brands of RWB’s choosing. The initial product offerings under the agreement will include body and face lotions, cosmetic creams, gels and serums, as well as soaps and bath bombs.
On August 19, 2020, RWB entered into the Critical 39 Agreement with Critical 39, a Spokane, Washington based company focused on delivering premium products throughout the United States.
-9-
Under terms of the Critical 39 Agreement, Critical 39 has already delivered 100,000 seeds to RWB’s 3.6 million square foot facility in Granville, Illinois where they are being cultivated in accordance with good agricultural practices and will be processed into finished whole hemp flower. The initial crop is expected to utilize a small portion of the facility’s capacity. The Critical 39 Agreement has provisions for the parties to extend the relationship into the year 2022.
On September 1, 2020, RWB entered into the Platinum Vape Definitive Agreement, the terms of which are described in the material change report dated September 8, 2020. The Platinum Vape acquisition was completed on September 14, 2020, as more fully described in the material change report dated September 15, 2020. See “Documents Incorporated by Reference”. Platinum Vape are purveyors of a full product line of premium cannabis products sold at more than 700 retailers throughout Michigan, California and Oklahoma. It is intended that Platinum Vape will operate as RWB Platinum Vape, a separate division of the Company focused on the current markets it serves as well as provide hemp-derived CBD-based products nationally in the United States. RWB Platinum Vape will operate in California as a THC licensed entity from a 5100 square foot facility in San Diego and is in the process of moving to a new 9000 square foot facility in Chula Vista, California. Outside of California, RWB Platinum Vape products are manufactured and sold through arm’s length entities in other markets. RWB Platinum Vape will retain the previous management team to oversee the day to day operations of RWB Platinum Vape and its expansion, when warranted, to other U.S. legal cannabis markets.
On September 11, 2020, RWB entered into the Convertible Debenture, the terms of which are described in the material change report dated September 15, 2020. See “Documents Incorporated by Reference”.
REGULATORY OVERVIEW
In accordance with Staff Notice 51-352, below is a discussion of the federal and state-level U.S. regulatory regimes in those jurisdictions where the Company is directly involved through certain subsidiaries and investees and expects to be directly involved through additional subsidiaries and investees in the U.S. legal cannabis industry. Pursuant to Staff Notice 51-352, issuers with U.S. cannabis-related activities are expected to clearly and prominently disclose certain prescribed information in prospectus filings and other required disclosure documents, such as this Prospectus. The Company is, through certain subsidiaries and investees, and intends to be, directly or indirectly, through additional subsidiaries and proposed acquisition targets, directly engaged in the cultivation, processing, sale and distribution of cannabis in the cannabis marketplaces in Michigan, Illinois, California and Massachusetts. The Company, through certain subsidiaries and investees, has limited indirect operations in Florida and Oklahoma through sublicensing arrangements with arm’s length entities. As such, the Company is subject to Staff Notice 51-352. Although the Company’s and, to the Company’s knowledge, its investees’ business activities are compliant with applicable U.S. state and local law, strict compliance with state and local laws with respect to cannabis may neither absolve the Company of liability under U.S. federal law, nor may it provide a defense to any federal proceeding which may be brought against the Company or its investees. In accordance with Staff Notice 51-352, the Company will evaluate, monitor and reassess this disclosure, and any related risks, on an ongoing basis and the same will be supplemented and amended to investors in public filings, including in the event of government policy changes or the introduction of new or amended guidance, laws or regulations regarding cannabis regulation. Any non-compliance, citations or notices of violation which may have an impact on the Company’s licenses, business activities or operations will be promptly disclosed by the Company.
In accordance with the Staff Notice 51-352, below is a table of concordance that is intended to assist readers in identifying those parts of this Prospectus that address the disclosure expectations outlined in Staff Notice 51-352.
Industry
| Specific Disclosure Necessary to Fairly
Present all Material Facts, Risks and
Uncertainties
| Cross-Reference (Prospectus or
Documents Incorporated by
Reference)
|
Involvement
|
All Issuers with
| Describe the nature of the issuer’s involvement in the U.S. marijuana industry and include the disclosures indicated for at least one of the direct,
| “The Company – Summary of the
|
U.S. Marijuana-
| Business” and “Recent
|
Related Activities
| Developments” in this Prospectus
|
-10-
| indirect and ancillary industry involvement types noted in this table.
| “General Development of the
Business – Three Year History” and
“Description of the Business” in the
Annual Information Form
“General Development of the
Business” and “Narrative
Description of the Business” in the
Listing Statement
|
Prominently state that marijuana is illegal under U.S. federal law and that enforcement of relevant laws is a significant risk
| Pages iv to vi (disclosure in bold typeface), “Regulatory Overview”
and “Risk Factors” in this
Prospectus
|
“Risk Factors – Risks Related to the Cannabis Industry” in the Annual Information Form
|
“Narrative Description of the
Business – Market Information,
Trends, Commitments, Events and Uncertainties”, “Risk Factors – Risks Related to the Cannabis Industry” in the Listing Statement
|
Discuss any statements and other available
guidance made by federal authorities or
prosecutors regarding the risk of enforcement
action in any jurisdiction where the issuer conducts
U.S. marijuana-related activities.
| Pages iv to vi (disclosure in bold
typeface), “Regulatory Overview”
and “Risk Factors” in this
Prospectus
“Risk Factors – Risks Related to the
Cannabis Industry” in the Annual
Information Form
“Narrative Description of the
Business – Market Information,
Trends, Commitments, Events and
Uncertainties” in the Listing
Statement
|
Outline related risks including, among others, the risk that third party service providers could suspend or withdraw services and the risk that regulatory bodies could impose certain restrictions on the issuer’s ability to operate in the U.S.
| “Risk Factors” in this Prospectus
|
“Risk Factors – Risks Related to the Cannabis Industry” in the Annual Information Form
|
“Narrative Description of the
Business – Market Information,
Trends, Commitments, Events and Uncertainties” and “Risk Factors – Risks Related to the Cannabis Industry” in the Listing Statement
|
Given the illegality of marijuana under U.S. federal law, discuss the issuer’s ability to access both
| “Risk Factors” in this Prospectus
|
-11-
| public and private capital and indicate what financing options are / are not available in order to
support continuing operations.
| “Risk Factors – Risks Related to the
Cannabis Industry” in the Annual
Information Form
|
“Risk Factors – Risks Related to the Cannabis Industry” in the Listing Statement
|
Quantify the issuer’s balance sheet and operating statement exposure to U.S. marijuana-related activities
| Please see the Interim Financial
Statements. As at June 30, 2020, the Company’s balance sheet and operating statement had 100% exposure to U.S. marijuana-related activities.
|
Disclose if legal advice has not been obtained, either in the form of a legal opinion or otherwise, regarding (a) compliance with applicable state
regulatory frameworks and (b) potential exposure
and implications arising from U.S. federal law.
| The Company and its subsidiaries have received and continue to receive legal input regarding (a)
compliance with applicable state
regulatory frameworks and (b)
potential exposure and implications
arising from U.S. federal law in certain respects.
|
U.S. Marijuana
Issuers with direct
involvement in cultivation or distribution
| Outline the regulations for U.S. states in which the issuer operates and confirm how the issuer
complies with applicable licensing requirements and the regulatory framework enacted by the applicable U.S. state.
| “Regulatory Overview” in this Prospectus
|
Discuss the issuer’s program for monitoring compliance with U.S. state law on an ongoing basis, outline internal compliance procedures and
provide a positive statement indicating that the
issuer is in compliance with U.S. state law and the related licensing framework. Promptly disclose any non-compliance, citations or notices of violation which may have an impact on the issuer’s licence, business activities or operations.
| “Regulatory Overview” in this
Prospectus
The Company has not received any
notices of non-compliance,
citations or notices of violation
which may have an impact on the
Company’s license, business
activities or operations.
|
Outline the regulations for U.S. states in which the issuer’s investee(s) operate.
| “Regulatory Overview” in this Prospectus
|
Provide reasonable assurance, through either positive or negative statements, that the investee’s
| “The Company – Summary of the
Business”, “Regulatory Overview”
and “Risk Factors” in this
Prospectus
|
business is in compliance with applicable licensing
|
requirements and the regulatory framework
|
enacted by the applicable U.S. state. Promptly
disclose any noncompliance, citations or notices of
|
“Description of the Business” and
“Risk Factors” in the Annual
|
violation, of which the issuer is aware, that may
|
have an impact on the investee’s licence, business
activities or operations.
| Information Form
|
| “Narrative Description of the
|
| Business” and “Risk Factors” in the
Listing Statement
|
-12-
|
| The Company’s investees have not
received any notices of non-
compliance, citations or notices of
violation which may have an impact on the investees’ license, business activities or operations.
|
U.S. Marijuana
Issuers with
material ancillary
involvement
| Provide reasonable assurance, through either positive or negative statements, that the applicable
customer’s or investee’s business is in compliance
with applicable licensing requirements and the regulatory framework enacted by the applicable U.S. state.
| “Regulatory Overview” in this Prospectus
|
U.S. Federal Regulatory Landscape
Under U.S. federal law, marijuana is currently classified as a Schedule I drug under the Controlled Substances Act. As such, the federal Drug Enforcement Agency (“DEA”) considers marijuana to have a high potential for abuse, no currently accepted medical use in treatment in the United States, and a lack of accepted safety for use of the drug under medical supervision.
State laws that permit and regulate the production, distribution and use of cannabis for adult-use or medical purposes are in direct conflict with the Controlled Substances Act, which makes cannabis use and possession federally illegal. Although certain states and territories of the U.S. authorize medical or adult-use cannabis production and distribution by licensed or registered entities, under U.S. federal law, the possession, use, cultivation, and transfer of cannabis and any related drug paraphernalia is illegal and any such acts are criminal acts under federal law under any and all circumstances under the Controlled Substances Act. Although the Company’s activities are believed to be compliant with applicable United States state and local law, strict compliance with state and local laws with respect to cannabis may neither absolve the Company of liability under United States federal law, nor may it provide a defense to any federal proceeding which may be brought against the Company.
As of August 25, 2020, 33 states and the District of Columbia have passed laws legalizing marijuana for medicinal use by eligible patients. In the District of Columbia, Puerto Rico, the U.S. Virgin Islands, the Northern Mariana Islands, and Guam and 11 of these states – Alaska, California, Colorado, Illinois, Maine, Massachusetts, Michigan, Nevada, Oregon, Vermont and Washington – marijuana is legal for adult-use regardless of medical condition, although Washington D.C. has not legalized commercial sale of cannabis.
As states increasingly legalized medical and/or adult-use cannabis, the federal government attempted to provide clarity on the incongruity between federal prohibition under the Controlled Substances Act and these state-legal regulatory frameworks. Until 2018, the federal government provided guidance to federal law enforcement agencies and banking institutions through a series of United States Department of Justice (“DOJ”) memoranda. The most recent such memorandum was drafted by former Deputy Attorney General James Cole in 2013 (the “Cole Memo”).
The Cole Memo offered guidance to federal enforcement agencies as to how to prioritize civil enforcement, criminal investigations and prosecutions regarding marijuana in all states. The memo put forth eight prosecution priorities:
1. Preventing the distribution of cannabis to minors;
2. Preventing revenue from the sale of cannabis from going to criminal enterprises, gangs and cartels;
3. Preventing the diversion of cannabis from states where it is legal under state law in some form to other states;
-13-
4. Preventing state-authorized cannabis activity from being used as a cover or pretext for the trafficking of other illegal drugs or other illegal activity;
5. Preventing the violence and the use of firearms in the cultivation and distribution of cannabis;
6. Preventing drugged driving and the exacerbation of other adverse public health consequences associated with cannabis use;
7. Preventing the growing of cannabis on public lands and the attendant public safety and environmental dangers posed by cannabis production on public lands; and
8. Preventing cannabis possession or use on federal property.
On January 4, 2018, then United States Attorney General Jeff Sessions rescinded the Cole Memo and a number of other cannabis-related memoranda by issuing a new memorandum to all United States Attorneys (the “Sessions Memo”). Rather than establish national enforcement priorities particular to marijuana-related crimes in jurisdictions where certain marijuana activity was legal under state law, the Sessions Memo instructs that “[i]n deciding which marijuana activities to prosecute... with the [DOJ’s] finite resources, prosecutors should follow the well-established principles that govern all federal prosecutions.” Namely, these include the seriousness of the offense, history of criminal activity, deterrent effect of prosecution, the interests of victims, and other principles.
Then U.S. Attorney General Jeff Sessions resigned on November 7, 2018 and was replaced by Matthew Whitaker as interim Attorney General. On February 14, 2019, William Barr was sworn in as Attorney General. It is unclear what position the new Attorney General will take on the enforcement of federal laws with regard to the U.S. cannabis industry. However, in a written response to questions from U.S. Senator Cory Booker made as a nominee, Attorney General Barr stated “I do not intend to go after parties who have complied with state law in reliance on the Cole Memorandum.”
Due to the Controlled Substances Act’s categorization of cannabis as a Schedule I drug, federal law also makes it illegal for financial institutions that depend on the Federal Reserve’s money transfer system to take any proceeds from marijuana sales as deposits. Banks and other financial institutions could be prosecuted and possibly convicted of money laundering for providing services to cannabis businesses under the United States Currency and Foreign Transactions Reporting Act of 1970 as amended by Title III of the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001 (the “Bank Secrecy Act”). Therefore, under the Bank Secrecy Act, banks or other financial institutions that provide a cannabis business with a checking account, debit or credit card, small business loan, or any other service could be charged with money laundering or conspiracy.
While there has been no change in U.S. federal banking laws to accommodate businesses in the large and increasing number of U.S. states that have legalized medical and/or adult-use marijuana, the Department of the Treasury Financial Crimes Enforcement Network (“FinCEN”), in 2014, issued guidance to financial institutions who may conduct transactions with cannabis-related businesses (the “FinCEN Guidance”). Issued simultaneously with the FinCEN Guidance was a memorandum from the Department of Justice that advised prosecutors not to focus their enforcement efforts on banks and other financial institutions that serve marijuana-related businesses so long as that business is legal in their state and none of the federal enforcement priorities referenced in the Cole Memo are being violated (such as keeping marijuana away from children and out of the hands of organized crime). This memorandum was also rescinded by former Attorney General Sessions on January 4, 2018. The FinCEN Guidance, however, remains in effect and clarifies how financial institutions can provide services to marijuana- related businesses consistent with their Bank Secrecy Act obligations, including thorough customer due diligence, but makes it clear that they are doing so at their own risk. Under the FinCEN Guidance, financial institutions must submit a suspicious activity report (“SAR”) in connection with all cannabis-related banking activities by any client of such financial institution, in accordance with federal money laundering laws. These cannabis-related SARs are divided into three categories – cannabis limited, cannabis priority, and cannabis terminated – based on the financial institution’s belief that the business in question follows state law, is operating outside of compliance with state law, or where the banking relationship has been terminated, respectively. With respect to information regarding state
-14-
licensure obtained in connection with such customer due diligence, a financial institution may reasonably rely on the accuracy of information provided by state licensing authorities, where states make such information available.
Because most banks and other financial institutions are unwilling to provide any banking or financial services to marijuana businesses, these businesses can be forced into becoming “cash-only” businesses. While the FinCEN Guidance decreased some risk for banks and financial institutions considering serving the industry, in practice it has not increased banks’ willingness to provide services to marijuana businesses. This is because, as described above, the current law does not guarantee banks immunity from prosecution, and it also requires banks and other financial institutions to undertake time-consuming and costly due diligence on each marijuana business they accept as a customer. In fact, some banks that had been servicing marijuana businesses have been closing the marijuana businesses’ accounts and are now refusing to open accounts for new marijuana businesses due to cost, risk, or both.
The few state-chartered banks and/or credit unions that have agreed to work with marijuana businesses are limiting those accounts to small percentages of their total deposits to avoid creating a liquidity risk. Since, theoretically, the federal government could change the banking laws as it relates to marijuana businesses at any time and without notice, these credit unions must keep sufficient cash on hand to be able to return the full value of all deposits from marijuana businesses in a single day, while also keeping sufficient liquid capital on hand to serve their other customers. Those state-chartered banks and credit unions that do have customers in the marijuana industry charge marijuana businesses high fees to pass on the added cost of ensuring compliance with the FinCEN Guidance.
Unlike the Cole Memo, however, the FinCEN Guidance from 2014 has not been rescinded. The Secretary of the U.S. Department of the Treasury, Stephen Mnuchin, has publicly stated that the Department was not informed of any plans to rescind the Cole Memo. Secretary Mnuchin stated that he does not have a desire to rescind the FinCEN Guidance.9
In recent years, certain temporary federal legislative enactments that protect the medical marijuana and hemp industries have also been in effect. For instance, certain marijuana businesses receive a measure of protection from federal prosecution by operation of temporary appropriations measures that have been enacted into law as amendments (or “riders”) to federal spending bills passed by Congress and signed by both Presidents Obama and Trump. For instance, in the Appropriations Act of 2015, Congress included the Rohrabacher-Farr Amendment that prohibits the DOJ from expending any funds to enforce any law that interferes with a state’s implementation of its own medical marijuana laws.
The Rohrabacher-Farr Amendment (now known colloquially as the “Joyce/Leahy Amendment” after its most recent sponsors) was included in the Fiscal Year Appropriations Bill, which was signed by President Trump on December
20, 2019 and funds the departments of the federal government through the fiscal year ending September 30, 2020. In signing the Act, President Trump issued a signing statement noting that the Act “provides that the Department of Justice may not use any funds to prevent implementation of medical marijuana laws by various states and territories,” and further stating “I will treat this provision consistent with the President’s constitutional responsibility to faithfully execute the laws of the United States.” While the signing statement can fairly be read to mean that the executive branch intends to enforce the Controlled Substances Act and other federal laws prohibiting the sale and possession of medical marijuana, the president did issue a similar signing statement in 2017 and no federal enforcement actions followed. The ongoing inclusion of the Rohrabacher-Farr Amendment in future U.S. legislation is subject to political change.
CBD is a product that often is derived from hemp, which contains only trace amounts of THC, the psychoactive substance found in marijuana. On December 20, 2018, President Trump signed the Agriculture Improvement Act of 2018 (popularly known as the 2018 Farm Bill) into law. Until the 2018 Farm Bill became law, hemp and products derived from it, such as CBD, fell within the definition of “marijuana” under the Controlled Substances Act and the DEA classified hemp as a Schedule I controlled substance because hemp is part of the cannabis plant.
The 2018 Farm Bill defines hemp as the plant Cannabis sativa L. and any part of the plant with a delta-9 THC concentration of not more than 0.3 percent by dry weight and removes hemp from the Controlled Substances Act. The 2018 Farm Bill also allows states to create regulatory programs allowing for the licensed cultivation of hemp and production of hemp-derived products. Hemp and products derived from it, such as CBD, may then be sold into
-15-
commerce and transported across state lines provided that the hemp from which any product is derived was cultivated under a license issued by an authorized state program and otherwise meets the definition of hemp removed from the Controlled Substances Act. Notwithstanding the 2018 Farm Bill, the FDA has maintained that infusion of CBD into food products and beverages or making health claims for hemp-derived products remains unlawful when introduced into interstate commerce pursuant to the U.S. federal Food, Drug and Cosmetic Act.
An additional challenge to marijuana-related businesses is that the provisions of the U.S. Internal Revenue Code, Section 280E, are being applied by the IRS to businesses operating in the medical and adult-use marijuana industry. Section 280E of the Internal Revenue Code prohibits marijuana businesses from deducting their ordinary and necessary business expenses, forcing them to pay higher effective federal tax rates than similar companies in other industries. The effective tax rate on a marijuana business depends on how large its ratio of non-deductible expenses is to its total revenues. Therefore, businesses in the U.S. legal cannabis industry may be less profitable than they would otherwise be.
Despite the recent rescission of the Cole Memo, the Company intends to implement the following to ensure compliance with the guidance provided by the Cole Memo, the FinCEN Guidance, and other best industry practices:
·The Company, its subsidiaries and its investees intend to operate in compliance with licensing requirements that are set forth with regards to cannabis operation by the applicable state, county, municipality, town, township, borough, and other political/administrative divisions;
·The Company’s, its subsidiaries’ and its investees’ cannabis-related activities will adhere to the scope of the licensing obtained;
·The Company, its subsidiaries and its investees will put in place procedures to perform due diligence on contractors or anyone provided access to secure areas of its facilities to prevent products from being distributed to minors;
·The Company, its subsidiaries and its investees will work to ensure that the licensed operators have an adequate inventory tracking system and adequate procedures in place so that their compliance system can track inventory effectively. This will be implemented to ensure that there is no diversion of cannabis or cannabis products into states where cannabis is not permitted by state law, or across state lines in general;
·The Company, its subsidiaries and its investees will conduct background checks as required by applicable state law;
·The Company, its subsidiaries and its investees will conduct reviews of activities of the cannabis businesses, the premises on which they operate, and the policies and procedures that are related to possession of cannabis or cannabis products outside of its licensed premises (including the cases where such possession is permitted by regulation – e.g. transfer of products between licensed premises). These reviews will ensure that licensed operators do not possess or use cannabis on federal property or engage in manufacturing or cultivation of cannabis on federal lands; and
·The Company’s, its subsidiaries’ and its investees’ product packaging will comply with applicable regulations and contain necessary disclaimers about the contents of the products to prevent adverse public health consequences from cannabis use and prevent impaired driving.
The following sections describe the legal and regulatory landscape in the states in which the Company currently or intends to, through its subsidiaries or investees, operate or distribute cannabis products or maintain sublicensing agreements with arm’s length entities to engage in cannabis activities: California, Florida, Illinois, Massachusetts, Michigan and Oklahoma. While the Company and its subsidiaries, to the Company’s knowledge, work to ensure that their operations comply with applicable state laws, regulations, and licensing requirements, for the reasons described above and the risks further described under the heading “Risk Factors”, there are significant risks associated with the business of the Company. Readers are strongly encouraged to carefully read and consider all
-16-
of the risk factors contained under the heading “Risk Factors” below and in the documents incorporated by reference in this Prospectus, including those risks identified and discussed under the heading “Risk Factors” in the Listing Statement, the Annual Information Form, the Annual Financial Statements and the Annual MD&A, all of which are incorporated by reference into this Prospectus.
California
California Regulatory Landscape
In 1996, California was the first state to effectively legalize medical marijuana through Proposition 215, the Compassionate Use Act of 1996. The Compassionate Use Act provided a defense to laws criminalizing the use, possession and cultivation of marijuana for medical patients with a physician recommendation for the use of medical cannabis in the treatment of cancer, anorexia, AIDS, chronic pain, spasticity, glaucoma, arthritis, migraine, or any other illness for which marijuana provides relief.
In 2003, Senate Bill 420 was signed into law establishing an optional identification card system for medical marijuana patients.
In September 2015, the California legislature passed three bills collectively known as the Medical Cannabis Regulation and Safety Act (“MCRSA”). The MCRSA established a licensing and regulatory framework for medical marijuana businesses in California. The system created multiple license types for dispensaries, infused products and concentrates manufacturers, cultivation facilities, testing laboratories, and distributors. Product manufacturers would require either volatile solvent or non-volatile solvent manufacturing licenses depending on their specific extraction methodology. Multiple agencies would oversee different aspects of the program and businesses would require a state license and local approval to operate. However in November 2016, voters in California overwhelmingly passed Proposition 64, the Adult-Use of Marijuana Act (“AUMA”) creating an adult-use marijuana program for adults 21 years of age or older. AUMA had some conflicting provisions with MCRSA, so in June 2017, the California State Legislature passed Senate Bill No. 94, known as the Medicinal and Adult-Use Cannabis Regulation and Safety Act (“MAUCRSA”), which amalgamates MCRSA and AUMA to provide a unified set of regulations to govern a medical and adult-use licensing regime for cannabis businesses in California. The four agencies that regulate marijuana at the state level are the Bureau of Cannabis Control (“BCC”), California Department of Food and Agriculture, California Department of Public Health, and California Department of Tax and Fee Administration. MAUCRSA went into effect on January 1, 2018.
One of the central features of MAUCRSA is known as “local control.” In order to legally operate a medical or adult- use marijuana business in California, an operator must have both a local and state license or permit. This requires license holders to operate in cities or counties with marijuana licensing programs. Cities and counties in California are allowed to determine the number of licenses they will issue to marijuana operators, or can choose to outright ban marijuana.
California Licensing Regime
Once an operator obtains local approval, the operator must obtain state licenses before conducting any commercial marijuana activity. There are 13 different license types that cover all commercial activity. License types 1-3 and 5 authorize the cultivation of medical and/or adult-use marijuana plants. Type 4 licenses are for nurseries that cultivate and sell clones and “teens” (immature marijuana plants that have established roots but require further vegetation prior to being sent into the flowering period). Type 6 and 7 licenses authorize manufacturers to process marijuana biomass into certain value-added products such as infused edibles, shatter or marijuana distillate oil with the use of volatile or non-volatile solvents, depending on the license type. Type 8 licenses are held by testing facilities who test samples of marijuana products and generate “certificates of analysis,” which include important information regarding the potency of products and whether products have passed or failed certain threshold tests for pesticide and microbiological contamination. Type 9 licenses are issued to “non-storefront” retailers, commonly called delivery services, who bring marijuana products directly to customers and patients at their residences or other chosen delivery location. Type 10 licenses are issued to storefront retailers, or dispensaries, which are open to the public and sell marijuana products onsite. Type 11 licenses are issued to distributors who move marijuana and marijuana products to all license types, including retailers, and ensure batch testing of all cannabis goods by
-17-
testing facilities prior to distribution. Type 12 licenses are issued to microbusinesses which allow the licensee to engage in at least three of four specified commercial cannabis business activities (i.e., cultivation in an area of less than 10,000 square feet, distribution, type 6 (non-volatile) manufacturing and retail). Type 13 licenses are known as “Transport-Only” distribution licenses, and they allow the distributor to transport marijuana and marijuana products between licensees, but not to retailers.
RWB Platinum Vape currently holds the following licenses through its subsidiary, Vista Prime Management LLC (as more fully described in “Regulatory Overview – Cannabis Licenses Held by State and Investee”):
·One annual manufacturing license (adult and medicinal) issued by the CDPH (as defined herein); and
·One medical distributor provisional license issued by the BCC.
The Company intends to submit a change of control application to applicable state regulatory authorities for the foregoing licenses to transfer ownership to RWB Platinum Vape.
California Agencies Regulating the Commercial Cannabis Industry
Three California state agencies are tasked with regulating the marijuana industry. The California Department of Food and Agriculture (“CDFA”) oversees nurseries and cultivators; the California Department of Public Health (“CDPH”) oversees manufacturers, and the BCC oversees distributors, retailers, delivery services, and testing laboratories. Operators apply to one or more of these agencies for their licenses and each agency has released specific regulations in respect of the types of businesses they oversee. The BCC has a number of regulations that apply to all licensees, but the CDFA and CDPH regulations only apply to the licensees under their purview.
California Recordkeeping and Reporting
In California, depending on a local government’s own marijuana ordinances, plants may be cultivated outdoors, using mixed-light methods, or fully indoors. Cultivators must initially acquire seeds, clones, teens, or other immature plants from nurseries.
The cultivation, processing, and movement of marijuana within the state is tracked by the METRC system, into which all licensees are required to input their track and trace data (either manually or using another software that automatically uploads to METRC). Immature plants are assigned a Unique Identifier number (UID), and this number follows the flowers and biomass resulting from that plant through the supply chain, all the way to the consumer. Each licensee in the supply chain is required to meticulously log any processing, packaging, and sales associated with that UID.
California Security Requirements
Each local government in California has its own security requirements for cannabis businesses, which usually include comprehensive video surveillance, intrusion detection and alarms, and limited access areas in the dispensary. The state also has similar security requirements, including that there be limited-access areas where only employees and other authorized individuals may enter. All licensee employees must wear employee badges. The limited access areas must be locked with “commercial-grade, non-residential door locks on all points of entry and exit to the licensed premises.”
Each licensed premises must have a digital video surveillance system that can “effectively and clearly” record images of the area under surveillance. Cameras must be “in a location that allows the camera to clearly record activity occurring within 20 feet of all points of entry and exit on the licensed premises.” The regulations list specific areas which must be under surveillance, including places where cannabis goods are weighed, packed, stored, loaded, and unloaded, security rooms, and entrances and exits to the premises. Retailers must record point of sale areas on the video surveillance system.
-18-
California Transportation
Marijuana in California may only be transported by licensed distributors. Some cultivators and manufacturers have their own distribution licenses, and others contract with third-party distributors. Distributors may or may not take possession of the marijuana and marijuana products. How this is evolving in California currently is that, similar to the alcohol distribution model, retailers are choosing from a portfolio of products carried by the distributors they work with. Brands are doing some direct marketing to retailers, but many brands target their marketing to distributors.
California Inspections
Distributors are the point in the supply chain where final quality assurance testing is performed on products before they go to a retailer. Retailers may not accept product without an accompanying certificate of analysis (“COA”). Distributors must hold product to be tested on their premises in “quarantine” and arrange for an employee of a licensed testing laboratory to come to their premises and obtain samples from any and all goods proposed to be shipped to a retailer. Marijuana and marijuana products are issued either a “pass” or “fail” by the testing laboratory. Under some circumstances, the BCC’s regulations allow for failing product to be “remediated” or to be re-labeled to more accurately reflect the COA.
U.S. Attorney Statements in California
California is divided into four federal districts, each with its own appointed United States Attorney.
The office of McGregor Scott, U.S. attorney for the Eastern District of California, issued the following statement in January 2018: “The cultivation, distribution and possession of marijuana has long been and remains a violation of federal law for all purposes,” and that the office “will evaluate violations of those laws in accordance with our district’s federal law enforcement priorities and resources.”
Florida
Florida Regulatory Landscape
In 2014, the Florida Legislature passed the Compassionate Use Act which was the first legal medical cannabis program in the state’s history. The original Compassionate Use Act only allowed for low-THC cannabis to be dispensed and purchased by patients suffering from cancer and epilepsy. In 2016, the Legislature passed the Right To Try Act which allowed for full potency cannabis to be dispensed to patients suffering from a diagnosed terminal condition. Also in 2016, the Florida Medical Marijuana Legalization Initiative was introduced by citizen referendum and passed with a 71.3% majority. This initiative amended the state constitution and mandated an expansion of the state’s medical cannabis program.
The Florida Medical Marijuana Legalization Initiative, Amendment 2 (“Amendment 2”), and the expanded qualifying medical conditions, became effective on January 3, 2017. The Florida Department of Health (“FDOH”), physicians, dispensing organizations, and patients are also subject to Article X Section 29 of the Florida Constitution and §381.986 of the Florida Statutes. On June 9, 2017, the Florida House of Representatives and Florida Senate passed respective legislation to implement the expanded program by replacing large portions of the existing Compassionate Use Act, which officially became law on June 23, 2017. The law regulating Amendment
2 provides licenses to operate as Medical Marijuana Treatment Centers (“MMTCs”) to all entities that held an
active, unrestricted licenses to cultivate, process, transport, and dispense low-THC cannabis or medical cannabis before July 1, 2017, as well as an additional 10 entities. The law also provides for another four licenses to be issued for every 100,000 patients added to the state’s medical marijuana registry and allows MMTCs to open an unlimited number of dispensaries.
Florida Licenses
Subsection 381.986(8)(a) of the State of Florida Statutes provides a vertically-integrated regulatory framework that requires licensed producers, through MMTCs, to cultivate, process and dispense medical cannabis in a vertically
-19-
integrated marketplace. Licenses issued by the FDOH may be renewed biennially so long as the license meets the requirements of the law and the license holder pays a renewal fee.
The license permits the sale of medical cannabis to qualified patients to treat certain medical conditions. In Florida, there is no state-imposed limitation on the permitted size of cultivation or processing facilities, nor is there a limit on the number of plants that may be grown.
Dispensaries may be located in any location throughout Florida as long as the local government has not issued a prohibition against MMTC dispensaries in their respective municipality. Provided there is not a ban, a dispensary may be located in a site zoned for a pharmacy so long as the location is greater than 500 feet from a public or private elementary, middle, or secondary school.
Florida Licensing Requirements
Licenses issued by the FDOH are renewed biennially so long as the licensee meets requirements of the law and pays a renewal fee. Provided that the requisite renewal fees are paid, the renewal application is submitted in a timely manner, and there are no material violations noted against the applicable license, a licensee would expect to receive the applicable renewed license in the ordinary course of business.
An MMTC license applicant must demonstrate that: (i) they have been registered to do business in Florida for the previous five years, (ii) they possess a valid certificate of registration issued by the Florida Department of Agriculture and Consumer Services, (iii) they have the technical and technological ability to cultivate and produce cannabis, including, but not limited to, low-THC cannabis, (iv) they have the ability to secure the premises, resources, and personnel necessary to operate as an MMTC, (v) they have the ability to maintain accountability of raw materials, finished products, and by-products to prevent diversion or unlawful access to or possession of these substances, (vi) they have an infrastructure reasonably required to dispense cannabis to registered qualified patients statewide or regionally as determined by the FDOH, (vii) they have the financial ability to maintain operations for the duration of the two-year approval cycle, including the provision of certified financial statements to the FDOH, (viii) its owners, officers, board members and managers have passed a Level II background screening, inclusive of fingerprinting, and ensure that a medical director is employed to supervise the activities of the MMTC, and (ix) they have a diversity plan and veterans plan accompanied by a contractual process for establishing business relationships with veterans and minority contractors and/or employees. Upon approval of the application by the FDOH, the applicant must post a performance bond of up to US$5,000,000, which may be reduced by meeting certain criteria such as a minimum patient count.
The Company has limited, indirect cannabis activities in Florida through High Times, which sublicenses its brand to arm’s length entities. None of the Company, its subsidiaries or investees currently have any cannabis licenses or pending cannabis license applications in Florida.
Florida Recordkeeping and Reporting
The FDOH requires that any licensee establish, maintain, and control a computer software tracking system that traces cannabis from seed to sale and allows real-time, 24-hour access by the FDOH to such data. The tracking system must allow for integration of other seed-to-sale systems and, at a minimum, include notification of when marijuana seeds are planted, when marijuana plants are harvested and destroyed, and when cannabis is transported, sold, stolen, diverted, or lost. Additionally, the FDOH also maintains a patient and physician registry and licensees must comply with requirements and regulations relative to providing required data or proof of key events to said system.
Florida Dispensary Requirements
An MMTC may not dispense more than a 70-day supply of cannabis within a 70-day period, except an MMTC may not dispense more than a 35-day supply of marijuana in a form for smoking within a 35-day period. The MMTC employee who dispenses the cannabis must enter into the registry his or her name or unique employee identifier. The MMTC must verify that: (i) the qualified patient and the caregiver, if applicable, each has an active registration in the registry and active and valid medical cannabis use registry identification card, (ii) the amount and type of
-20-
cannabis dispensed matches the physician certification in the registry for the qualified patient, and (iii) the physician certification has not already been filled. An MMTC may not dispense to a qualified patient younger than 18 years of age, only to such patient’s caregiver. An MMTC may not dispense or sell any other type of cannabis, alcohol, or illicit drug-related product, except a cannabis delivery device as specified in the physician certification. An MMTC must, upon dispensing, record in the registry: (i) the date, time, quantity and form of cannabis dispensed, (ii) the type of cannabis delivery device dispensed, and (iii) the name and registry identification number of the qualified patient or caregiver to whom the cannabis delivery device was dispensed. An MMTC must ensure that patient records are not visible to anyone other than the patient, caregiver, and MMTC employees.
Florida Security Requirements
Each MMTC must maintain a video surveillance system that records continuously 24 hours per day and has specified features. MMTCs must retain video surveillance recordings for at least 45 days, or longer upon the request of law enforcement.
An MMTC’s outdoor premises must have sufficient lighting from dusk until dawn. An MMTC’s dispensing facilities must include a waiting area with sufficient space and seating to accommodate qualified patients and caregivers and at least one private consultation area and such facilities may not display products or dispense cannabis or cannabis delivery devices in the waiting area and may not dispense cannabis from its premises between the hours of 9:00 p.m. and 7:00 a.m. but may perform all other operations and deliver cannabis to qualified patients 24-hours a day.
Cannabis must be stored in a secured, locked room or a vault. An MMTC must have at least two employees, or two employees of a security agency, on the premises at all times where cultivation, processing, or storing of cannabis occurs. MMTC employees must wear a photographic identification badge and visitors must wear a visitor pass at all times on the premises. An MMTC must report to law enforcement within 24 hours after the MMTC is notified of or becomes aware of the theft, diversion or loss of cannabis.
Florida Transportation
A cannabis transportation manifest must be maintained in any vehicle transporting cannabis or a cannabis delivery device. The manifest must be generated from the MMTC’s seed-to-sale tracking system and must include the: (i) departure date and time, (ii) name, address, and license number of the originating MMTC, (iii) name and address of the recipient, (iv) quantity and form of any cannabis or cannabis delivery device being transported, (v) arrival date and time, (vi) delivery vehicle make and model and license plate number; and (vii)name and signature of the MMTC employees delivering the product. Further, a copy of the transportation manifest must be provided to each individual, MMTC that receives a delivery. MMTCs must retain copies of all cannabis transportation manifests for at least three years. Cannabis and cannabis delivery devices must be locked in a separate compartment or container within the vehicle and employees transporting cannabis or cannabis delivery devices must have their employee identification on them at all times. Lastly, at least two people must be in a vehicle transporting cannabis or cannabis delivery devices, and at least one person must remain in the vehicle while the cannabis or cannabis delivery device is being delivered.
Florida Inspections
The FDOH conducts announced and unannounced inspections of MMTCs to determine compliance with the laws and rules. The FDOH shall inspect an MMTC upon receiving a complaint or notice that the MMTC has dispensed cannabis containing mold, bacteria, or other contaminants that may cause an adverse effect to humans or the environment. The FDOH shall conduct at least a biennial inspection of each MMTC to evaluate the MMTC’s records, personnel, equipment, security, sanitation practices, and quality assurance practices.
-21-
Illinois
Illinois Regulatory Landscape
The Compassionate Use of Medical Cannabis Pilot Program Act (the “IL Act”) was signed into law in August 2013 and took effect on January 1, 2014. The IL Act provides medical cannabis access to registered patients who suffer from a list of over 40 medical conditions including epilepsy, cancer, HIV/AIDS, Crohn’s disease and post-traumatic stress disorder. As of April 9, 2019, approximately 61,200 patients have been registered under the IL Act and are qualified to purchase cannabis and cannabis products from registered dispensaries. On August 12, 2019 changes to this IL Act were effective, including making the medical program a permanent program and removing the pilot designation attached to the program. In addition to making the program permanent, new conditions were added as qualifying conditions.
Illinois Licensing Regime
The IL Act requires prospective cannabis business license holders to adhere to a thorough application process. Applicants for cannabis business licenses must meet, among others, the following requirements: (a) the proposed location for a dispensary must be suitable for public access, (b) the proposed location must not pose a detrimental impact to the surrounding community, (c) demonstrate compliance with safety procedures for dispensary employees, patients, and caregivers, and safe delivery and storage of cannabis and currency, (d) provide an adequate plan for recordkeeping, tracking and monitoring inventory, quality control, destruction and disposal of cannabis, and procedures to discourage unlawful activity, (e) develop a business plan specifying products to be sold, and (f) demonstrate knowledge of, experience, and proven record of ensuring optimal safety and accuracy in the dispensing and sale of cannabis. Once a license is granted, licensees have a continuing obligation to ensure no cannabis is sold, delivered, transported, or distributed to a location outside of Illinois.
Under the IL Act, dispensary, grower, and processing licenses are valid for one year. After the initial term, licensees are required to submit renewal applications. Pursuant to the IL Act, registration renewal applications must be received 45 days prior to expiration and may be denied if the licensee has a history of non-compliance and penalties.
MAG current holds one hemp grower license and one hemp processor license in Illinois. The Company does not, directly or indirectly, currently conduct any cannabis-related activities in Illinois. MAG is awaiting a response from regulatory authorities regarding a pending cannabis license application for a craft cannabis grower license that it applied for on March 17, 2020.
Illinois Agencies Regulating the Commercial Cannabis Industry
Oversight and implementation under the IL Act are divided among three Illinois state departments: the Department of Public Health (the “IL DPH”), the Department of Agriculture (the “IL DA”), and the Department of Financial and Professional Regulation (the “IL DFPR”). The IL DPH oversees the following IL Act mandates: (a) establish and maintain a confidential registry of caregivers and qualifying patients authorized to engage in the medical use of cannabis, (b) distribute educational materials about the health risks associated with the abuse of cannabis and prescription medications, (c) adopt rules to administer the patient and caregiver registration program, and (d) adopt rules establishing food handling requirements for cannabis-infused products that are prepared for human consumption. It is the responsibility of the IL DA to enforce the provisions of the IL Act relating to the registration and oversight of cultivation centers and the responsibility of the IL DFPR to enforce the provisions of the IL Act relating to the registration and oversight of dispensing organizations. The IL DPH, IL DA and IL DFPR may enter into inter-governmental agreements, as necessary, to carry out the provisions of the IL Act.
On June 25, 2019, Illinois Gov. J.B. Pritzker signed into law the Cannabis Regulation & Tax Act, which as of January 1, 2020, permits persons 21 years of age or older to possess, use, and purchase limited amounts of cannabis for personal/recreational use, beginning on January 1, 2020. In summary the act allows possession for Illinois residents of no more than 30 grams of cannabis flower; 5 grams of cannabis concentrate; no more than 500 milligrams of THC contained in a cannabis infused product; and patients registered under the medical cannabis program may possess more than 30 grams of cannabis if it is grown in their residence, in a secure location under
-22-
the conditions set forth in the IL Act. The IL Act also contains possession limits for non-Illinois residents. The Act IL contains very clear social equity language and requirements in order to qualify for a license.
Illinois has issued a limited amount of dispensary, producer/grower, and processing licenses. There are currently over 60 licensed dispensaries and 22 licensed cultivators.
Illinois Recordkeeping and Reporting
Illinois uses the BioTrack THC T&T system to manage the flow of reported data between each licensee and the state. NCC also uses the T&T system to ensure all reporting requirements are met. Information processed through the T&T system must be maintained in a secure location at the dispensing organization for five years.
Licensees are mandated by the IL Act to maintain records electronically and make them available for inspection by the IL DFPR upon request. Records that must be maintained and made available, as described in the IL Act, include: (a) operating procedures, (b) inventory records, policies, and procedures, (c) security records, and (d) staffing plans. All dispensing organization records, including business records such as monetary transactions and bank statements, must be kept for a minimum of three years. Records of destruction and disposal of all cannabis not sold, including notification to the IL DFPR and State Police, must be retained at the dispensary organization for a period of not less than five years.
Illinois Inventory and Storage
An organization’s agent-in-charge has primary oversight of the dispensing organization’s medical cannabis inventory control system. Under the IL Act, a dispensary’s inventory control system must be real-time, web-based, and accessible by the IL DFPR 24 hours a day, seven days a week. The T&T system used by NCC complies with such requirements.
The inventory control system of a dispensing organization must record all cannabis sales, waste, and acquisitions. Specifically, the inventory system must track and reconcile through the T&T system each day’s cannabis beginning inventory, acquisitions, sales, disposal and ending inventory. Tracked information must include (a) product descriptions including the quantity, strain, variety and batch number of each product received, (b) the name and registry identification number of the permitted cultivation center providing the medical cannabis, (c) the name and registry identification number of the permitted cultivation center agent delivering the medical cannabis, (d) the name and registry identification number of the dispensing organization agent receiving the medical cannabis, and (e) the date of acquisition. Dispensary managers are tasked with conducting and documenting monthly audits of the dispensing organization’s daily inventory according to generally accepted accounting principles.
The inventory control system of a cultivator and processing organization must conduct a weekly inventory of cannabis stock, which includes at a minimum, the date of the inventory, a summary of the inventory findings, the name, signature and title of the individuals who conducted the inventory and the agent-in-charge who oversaw the inventory, and the product name and quantity of cannabis plants or cannabis-infused products at the facility. The record of all medical cannabis sold must include the date of sale, the name of the dispensary facility to which the medical cannabis was sold and the batch number, product name and quantity of cannabis sold.
Storage of cannabis and cannabis product inventory is also regulated by the IL Act. Inventory must be stored on the dispensary’s licensed premises in a restricted access area. Appropriate storage temperatures, containers, and lighting are required to ensure the quality and purity of cannabis inventory is not adversely affected.
Illinois Security Requirements
Under the IL Act, dispensaries must implement security measures to deter and prevent entry into and theft from restricted access areas containing either cannabis or currency. Mandated security measures include security systems, panic alarms, and locked doors or barriers between the facility’s entrance and limited access areas. Admission to the limited access areas must be restricted to only registered qualifying patients, designated caregivers, principal officers, and agents conducting business with the dispensing organization. Visitors and persons conducting business with the licensee in limited access areas must always wear identification badges and
-23-
be escorted by a licensee’s agent authorized to enter the restricted access area. A visitor’s log must be kept on- site and be maintained for five years.
The IL Act states 24-hour video surveillance of both a licensee’s interior and exterior are required to be taken and kept for at least 90 days. Unless prohibited by law, video of all interior dispensary areas, including all points of entry and exit, safes, sales areas, and storage areas must be kept. Unobstructed video of the exterior perimeter, including the storefront, grow facility and the parking lot, must also be kept. Video surveillance cameras are required to be angled to allow for facial recognition and the capture of clear and certain identification of any person entering or exiting the dispensary area. Additionally, all video must be taken in lighting sufficient for clear viewing during all times of night or day. The IL Act also requires all security equipment to be inspected and tested within regular 30-day intervals.
Illinois Transportation
Prior to transporting any cannabis or cannabis-infused product, a cultivation facility must:
·Complete a shipping manifest using a form prescribed by the IL DA; and
·Securely transmit a copy of the manifest to the dispensary facility that will receive the products and to the IL DA before the close of business the day prior to transport. The manifest must be made available to the Illinois State Police upon request.
The cultivation facility shall maintain all shipping manifests and make them available at the request of the IL DA. Cannabis products that are being transported shall:
·Only be transported in a locked, safe and secure storage compartment that is part of the motor vehicle transporting the cannabis, or in a locked storage container that has a separate key or combination pad; and
·Not be visible from outside the motor vehicle.
Any motor vehicle transporting cannabis is required travel directly from the cultivation facility to the dispensary facility, or a testing laboratory, and must not make any stops in between except to other dispensary facilities or laboratories, for refueling or in case of an emergency. A cultivation center shall ensure that all delivery times and routes are randomized. A cultivation center shall staff all transport motor vehicles with a minimum of two employees. At least one delivery team member shall remain with the motor vehicle at all times that the motor vehicle contains cannabis. Each delivery team member shall have access to a secure form of communication with personnel at the cultivation center and the ability to contact law enforcement through the 911 emergency system at all times that the motor vehicle contains cannabis. Each delivery team member shall possess his or her department issued identification card at all times when transporting or delivering cannabis and shall produce it for the IL DA or IL DA’s authorized representative or law enforcement official upon request.
Illinois Inspections
Dispensing organizations are subject to random and unannounced dispensary inspections and cannabis testing by the IL DFPR and Illinois State Police. The IL DFPR and its authorized representatives may enter any place, including a vehicle, in which cannabis is held, stored, dispensed, sold, produced, delivered, transported, manufactured or disposed of and inspect in a reasonable manner, the place and all pertinent equipment, containers and labeling, and all materials, data and processes, and inventory any stock of cannabis and obtain samples of any cannabis or cannabis product, any labels or containers for cannabis, or paraphernalia.
The IL DFPR may conduct an investigation of an applicant, application, dispensing organization, principal officer, dispensary agent, third party vendor or any other party associated with a dispensing organization for an alleged violation of the IL Act or to determine qualifications to be granted a registration by the IL DFPR. The IL DFPR may
-24-
require an applicant or dispensing organization to produce documents, records or any other material pertinent to the investigation of an application or alleged violations of the IL Act.
Medical cannabis cultivation centers are also subject to random inspections by the IL DA.
Massachusetts
Massachusetts Regulatory Landscape
The Commonwealth of Massachusetts has authorized the cultivation, possession and distribution of marijuana for medical purposes by certain licensed Massachusetts marijuana businesses. The Medical Use of Marijuana Program (the “MUMP”) registers qualifying patients, personal caregivers, Marijuana Treatment Centers (“MTCs”), and MTC agents. The MUMP was established by Chapter 369 of the Acts of 2012, “An Act for the Humanitarian Medical Use of Marijuana”, following the passage of the Massachusetts Medical Marijuana Initiative, Ballot Question 3, in the 2012 general election. This act was repealed by Chapter 55 of the Acts of 2017. Additional statutory requirements governing the MUMP were enacted by the Legislature in 2017 and codified at G.L. c. 94I, et. seq. MTC Certificates of Registration are vertically integrated licenses in that each MTC Certificate of Registration entitles a license holder to one cultivation facility, one processing facility and one dispensary locations.
The Commonwealth of Massachusetts has also authorized the cultivation, possession, and use of marijuana by adults 21 years of age and older, as well as the licensure of certain marijuana-related businesses, through Chapter 334 of the Acts of 2016, “The Regulation and Taxation of Marijuana Act,” following the passage of Ballot Question 4 in the 2016 general election. Additional statutory requirements were enacted by the Legislature in 2017 and codified at G.L. c. 94g, et seq.
The Commonwealth of Massachusetts Cannabis Control Commission (“CCC”) regulations, 935 CMR 501.000 et seq. (“Massachusetts Medical Regulations”), provide a regulatory framework that requires MTCs to cultivate, process, transport and dispense medical cannabis in a vertically integrated marketplace. Patients with debilitating medical conditions qualify to participate in the program, including conditions such as cancer, glaucoma, positive status for HIV, AIDS, hepatitis C, ALS, Crohn’s disease, Parkinson’s disease, and MS when such diseases are debilitating, and other debilitating conditions as determined in writing by a qualifying patient’s healthcare provider. The CCC assumed control of the MUMP from the Department of Public Health on December 23, 2018.
The CCC regulations, 935 CMR 500.000 et seq. (“Massachusetts Adult Use Regulations”), provide a regulatory framework for “marijuana establishments,” which includes: (a) “Marijuana Cultivator” licenses, whether indoor or outdoor, (b) “Craft Marijuana Cooperative” licenses, (c) “Marijuana Product Manufacturer” licenses, (d) “Marijuana Microbusiness” licenses, (e) “Independent Testing Laboratory” licenses, (f) “Marijuana Retailer” licenses, (g) “Marijuana Transporter” licenses, (h) “Delivery-only Licensee”, (i) “Marijuana Research Facility” licenses, (j) “Social Consumption Establishment” licenses, and (k) any other type of licensed marijuana-related business, except a MTC.
The CCC regulations, 935 CMR 502.000 et seq., provide a regulatory framework for MTCs who are also licensed to conduct adult-use operations as a Cultivator, Product Manufacturer and Retailer.
Massachusetts Licensing Regime
The Massachusetts Medical Regulations delineate the licensing requirements for MTCs in Massachusetts. Licensed entities must demonstrate the following: (i) they are licensed and in good standing with the Secretary of the Commonwealth of Massachusetts; (ii) no executive, member or any entity owned or controlled by such executive or member directly or indirectly controls more than three MTC licenses; (iii) an MTC may not cultivate and dispense medical cannabis from more than two locations statewide; (iv) dispensary agents must be registered with the Massachusetts Department; (v) an MTC must have a program to provide reduced cost or free marijuana to patients with documented verifiable financial hardships; (vi) one executive of an MTC must register with the Massachusetts Department of Criminal Justice Information Services on behalf of the entity as an organization user of the Criminal Offender Record Information (iCORI) system; (vii) the MTC applicant has at least $500,000 in its
-25-
control as evidenced by bank statements, lines of credit or equivalent for its first application, and $400,000 for subsequent application; and (viii) payment of the required application fee.
After November 1, 2019, in an MTC application, an applicant must submit the following: (i) an application of intent, including, among other documentation, proof that the applicant is registered to do business in Massachusetts, a list of all persons or entities having direct or indirect control over the applicant, and documentation of the amounts and sources of capital resources available to the applicant; (ii) information in order to allow the State to conduct a background check for certain individuals affiliated with the applicant, and (iii) a management and operations profile, including, among other documentation, the applicant’s entity formation documents, a proposed timeline regarding when the marijuana establishment will be operational, and a detailed summary of operating policies and procedures for the applicant.
The CCC shall review applications from applicants in the order they were submitted as determined by the CCC’s electronic licensing system. The CCC shall grant or deny a provisional license not later than 90 days following notification to the applicant that all required packets are considered complete. On selection by the CCC, an applicant shall submit the required License fee and subsequently be issued a provisional license to develop an MTC, in the name of the entity. An MTC shall construct its facilities in accordance with the Massachusetts Medical Regulations, conditions set forth by the CCC in its provisional License and architectural review, and any applicable state and local laws, regulations, permits or licenses. On completion of all inspections required by the Commission, an MTC is eligible for a final License.
The Massachusetts Adult Use Regulations delineate the licensing requirements for marihuana establishments in Massachusetts. Licensed entities must submit the following: (i) an application of intent, including, among other documentation, proof that the applicant is registered to do business in Massachusetts, a list of all persons or entities having direct or indirect control over the applicant, and documentation of the amounts and sources of capital resources available to the applicant; (ii) information in order to allow the State to conduct a background check for certain individuals affiliated with the applicant, and (iii) a management and operations profile, including, among other documentation, the applicant’s entity formation documents, a proposed timeline regarding when the marijuana establishment will be operational, and a detailed summary of operating policies and procedures for the applicant.
Upon the determination by the CCC that a marihuana establishment applicant has responded to the application requirements in a satisfactory fashion, a copy of the completed application, to the extent permitted by law, will be forwarded to the municipality in which the marijuana establishment will be located. The CCC shall request that the municipality respond within 60 days of the date of the correspondence that the applicant’s proposed marijuana establishment is in compliance with municipal bylaws or ordinances. On selection by the CCC, an applicant shall submit the required license fee and subsequently be issued a provisional license to develop a marijuana establishment, in the name of the entity. On completion of all inspections required by the CCC, a marijuana establishment is eligible for a final license.
The Company does not, directly or indirectly, currently conduct any cannabis-related activities in Massachusetts. The Company submitted a change of control application to the CCC in September 2020 for the 2 cultivation licenses and 1 product manufacturing license to be acquired pursuant to the TDMA MIPA and is awaiting approval.
Massachusetts Dispensary Requirements
An MTC shall follow its written and approved operation procedures in the operation of its dispensary locations. Operating procedures shall include (i) security measures in compliance with the Massachusetts Regulations; (ii) employee security policies including personal safety and crime prevention techniques; (iii) hours of operation and after-hours contact information; (iv) storage and waste disposal policies;; (v) storage protocols in compliance with state law; (vi) a description of the various strains of marijuana that will be cultivated and dispensed, and the forms that will be dispensed; (vii) a price list for marijuana and MIPs, as well as an alternate price list for patients with documented verified financial hardship; (viii) procedures to ensure accurate recordkeeping including inventory protocols; (ix) plans for quality control; (x) a staffing plan and staffing records; (xi) emergency procedures; (xii) alcohol, smoke, and drug free workplace policies; (xiii) a plan to describe how confidential information will be maintained; (xiv) a policy for the immediate dismissal of an MTC agent who violates laws or regulations; (xv) a list
-26-
of all board members and executives; (xvi) policies and procedures for the handling of cash on the premises; (xvii) the standards and procedures by which the MTC determines the price it charges for marijuana; (xviii) policies and procedures for energy efficiency and conservation; and (xix) policies and procedures to promote workplace safety.
A marijuana retailer shall follow its written and approved operation procedures in the operation of its dispensary locations. Operating procedures shall include (i) security measures in compliance with the Massachusetts Adult Use Regulations; (ii) employee security policies including personal safety and crime prevention techniques; (iii) hours of operation and after-hours contact information; (iv) storage and waste disposal protocols in compliance with state law; (v) a description of the various strains of marijuana that will be cultivated and dispensed, and the forms that will be dispensed; (vi) price list for marijuana and marijuana products and alternate price lists for patients with verified financial hardship; (vii) procedures to ensure accurate recordkeeping including inventory protocols; (viii) plans for quality control; (ix) plans for quality control, including product testing for contaminants; (x) a staffing plan and staffing records; (xi) diversion identification and reporting protocols; (xii) emergency procedures, including a disaster plan; (xiii) alcohol, smoke, and drug-free workplace policies; (xiv) a plan describing how confidential information and other records will be maintained confidentially; (xv) policies related to the immediate dismissal of certain marijuana establishment agents who violate laws or regulations; (xvi) a list of all board members and executives of the licensee; (xvii) policies and procedures for the handling of cash on premises including storage, collection frequency and transport to financial institutions; (xviii) policies and procedures to prevent the diversion of marijuana to anyone younger than 21 years old; policies and procedures for energy efficiency and conservation; and (xiv) policies and procedures to promote workplace safety.
Massachusetts Security Requirements
An MTC shall implement sufficient security measures to deter and prevent unauthorized entrance into areas containing marijuana and theft of marijuana. These measures must include: (i) allowing only registered qualifying patients, caregivers, dispensary agents, authorized persons, or approved outside contractors access to the MTC facility; (ii) preventing individuals from remaining on the premises of an RMD if they are not engaging in activities that are permitted; (iii) disposing of marijuana or byproducts in compliance with law; (iv) securing all entrances to the MTC to prevent unauthorized access; (v) establishing limited access areas accessible only to authorized personnel; (vi) storing finished marijuana in a secure locked safe or vault; (vii) keeping all locks and security equipment in good working order; (viii) prohibiting keys from being left in locks or stored in an accessible area; (ix) prohibiting accessibility of security measures to persons other than authorized personnel; (x) ensuring that the outside perimeter of the MTC is sufficiently lit to facilitate surveillance; (xi) ensuring that all marijuana products are kept out of plain sight; (xii) developing emergency policies and procedures for securing all product; (xiii) developing sufficient addition safeguards as required by the CCC; (xiv) sharing the MTC’s floorplan or layout with law enforcement authorities; and (xv) inside the MTC, keeping all marijuana within a limited access area.
A marijuana establishment shall implement sufficient security measures to deter theft of marijuana and marijuana products, prevent unauthorized entrance into areas containing marijuana and marijuana products, and ensure the safety of employees, consumers and the general public. These measures must include: (i) positively identifying individuals seeking access to the premises of the marijuana establishment or to whom or marijuana products are being transported in order to limit access solely to individuals 21 years of age or older; (ii) adopting procedures to prevent loitering and ensure that only individuals engaging in activity expressly or by necessary implication are allowed to remain on the premises; (iii) disposing of Marijuana in accordance with the Massachusetts Adult Use Regulations in excess of the quantity required for normal, efficient operation; (iv) securing all entrances to the marijuana establishment to prevent unauthorized access; (v) establishing limited access areas, which shall be accessible only to specifically authorized personnel; (vi) storing all marijuana products in a secure, locked safe or vault in such a manner as to prevent diversion, theft and loss; (vii) keeping all safes, vaults, and any other equipment or areas used for the production, cultivation, harvesting, processing or storage, including prior to disposal, of marijuana or marijuana products securely locked and protected from entry, except for the actual time required to remove or replace marijuana; (viii) keeping all locks and security equipment in good working order; (ix) prohibiting keys, if any, from being left in the locks or stored or placed in a location accessible to persons other than specifically authorized personnel; (x) prohibiting accessibility of security measures such as combination numbers, passwords or electronic or biometric security systems, to persons other than specifically authorized personnel; (xi) ensuring that the outside perimeter of the marijuana establishment is sufficiently lit to facilitate surveillance, where applicable; (xii) ensuring that all marijuana products are kept out of plain sight and are not
-27-
visible from a public place, outside of the marijuana establishment, without the use of binoculars, optical aids or aircraft; (xiii) developing emergency policies and procedures for securing all product following any instance of diversion, theft or loss of marijuana, and conduct an assessment to determine whether additional safeguards are necessary; (xiv) developing sufficient additional safeguards as required that present special security concerns; (xv) at marijuana establishments where transactions are conducted in cash, establishing procedures for safe cash handling and cash transportation to financial institutions to prevent theft, loss and associated risks to the safety of employees, customers and the general public; (xvi) sharing the marijuana establishment’s floor plan or layout of the facility with law enforcement authorities, and in a manner and scope as required by the municipality and identifying when the use of flammable or combustible solvents, chemicals or other materials are in use at the marijuana establishment; and (xvii) sharing the marijuana establishment’s security plan and procedures with law enforcement authorities, including police and fire departments, in the municipality where the marijuana establishment is located and periodically updating law enforcement authorities, police and fire departments, if the plans or procedures are modified in a material way.
Massachusetts Transportation
Marijuana or marijuana-infused products (“MIPs”) may only be transported by marijuana transporters or MTC agents on behalf of a MTC between separately-owned MTCs, a patient or his or her caregiver, or a laboratory in compliance with the Massachusetts Medical Regulations. An MTC shall staff transport vehicles with a minimum of two MTC agents. At least one MTC agent shall remain with the vehicle when the vehicle contains marijuana or MIPs. Prior to leaving the origination location, an MTC must weigh, inventory, and account for, on video, the marijuana to be transported.
Marijuana must be packaged in sealed, labeled, and tamper-proof packaging prior to and during transportation. In the case of an emergency stop, a log must be maintained describing the reason for the stop, the duration, the location, and any activities of personnel exiting the vehicle. An MTC shall ensure that delivery times and routes are randomized. Each dispensary agent shall carry his or her CCC-issued agent registration card when transporting marijuana or MIPs and shall produce it to CCC representatives or law enforcement officials upon request. Where videotaping is required when weighing, inventorying, and accounting of marijuana before transportation or after receipt, the video must show each product being weighed, the weight, and the manifest. An MTC must document and report any unusual discrepancy in weight or inventory to the CCC and local law enforcement within 24 hours. An MTC shall report to the CCC and local law enforcement any vehicle accidents, diversions, losses, or other reportable incidents that occur during transport, within 24 hours. An MTC shall retain transportation manifests for no less than one year and make them available to the CCC upon request. Any cash received from a qualifying patient or personal caregiver must be transported to an MTC immediately upon completion of the scheduled deliveries. Vehicles used in transportation must be owned, leased or rented by the MTC, be properly registered, and contain a GPS system that is monitored by the MTC during transport of marijuana and said vehicle must be inspected and approved by the CCC prior to use.
During transit, an MTC shall ensure that: (i) marijuana or MIPs are transported in a secure, locked storage compartment that is part of the vehicle; (ii) the storage compartment cannot be easily removed (for example, bolts, fittings, straps or other types of fasteners may not be easily accessible and not capable of being manipulated with commonly available tools); and (iii) product is transported in a vehicle that bears no markings indicating that the vehicle is being used to transport marijuana or MIPs and does not indicate the name of the MTC. Each MTC agent transporting marijuana or MIPs shall have access to a secure form of communication with personnel at the origination location when the vehicle contains marijuana or MIPs.
Marijuana or MIPs may only be transported by marihuana establishment agents on behalf of a marihuana establishment between separately-owned marihuana establishments in compliance with the Massachusetts Adult Use Regulations. A licensed marijuana transporter may contract with a licensed marijuana establishment to transport that licensee’s marijuana products to other licensed marijuana establishments.
Marijuana must be packaged in sealed, labeled, and tamper-proof packaging prior to and during transportation. In the case of an emergency stop, a log must be maintained describing the reason for the stop, the duration, the location, and any activities of personnel exiting the vehicle. The originating and receiving licensed marijuana establishments shall ensure that all transported marijuana products are linked to the seed-to-sale tracking program.
-28-
Prior to leaving a marijuana establishment for the purpose of transporting marijuana products, the originating marijuana establishment must weigh, inventory, and account for, on video, all marijuana products to be transported. Within eight hours after arrival at the destination marijuana establishment, the destination establishment must reweigh, re-inventory, and account for, on video, all marijuana products transported A marihuana establishment shall report to the CCC and local law enforcement any vehicle accidents, diversions, losses, or other reportable incidents that occur during transport, within 24 hours. An marihuana shall retain transportation manifests for no less than one year and make them available to the CCC upon request. Vehicles used in transportation must be owned, leased or rented by the marihuana establishment or marihuana transporter, be properly registered, and contain a GPS system that is monitored by the marihuana establishment during transport of marijuana and said vehicle must be inspected and approved by the CCC prior to use.
During transit, a marijuana establishment or marijuana transporter shall ensure that: (i) marijuana or MIPs are transported in a secure, locked storage compartment that is part of the vehicle; (ii) the storage compartment cannot be easily removed (for example, bolts, fittings, straps or other types of fasteners may not be easily accessible and not capable of being manipulated with commonly available tools); and (iii) product is transported in a vehicle that bears no markings indicating that the vehicle is being used to transport marijuana or MIPs and does not indicate the name of the marihuana establishment. Each marihuana establishment agent transporting marijuana or MIPs shall have access to a secure form of communication with personnel at the origination location when the vehicle contains marijuana or MIPs.
Massachusetts Inspections
The CCC or its agents may inspect an MTC, marijuana establishment, and affiliated vehicles at any time without prior notice. An MTC or marijuana establishment shall immediately upon request make available to the CCC information that may be relevant to a CCC inspection, and the CCC may direct an MTC or marijuana establishment to test marijuana for contaminants. Any violations found will be noted in a deficiency statement that will be provided to the MTC or marijuana establishment, and the MTC or marijuana establishment shall thereafter submit a Plan of Correction to the CCC outlining with particularity each deficiency and the timetable and steps to remediate the same. The CCC shall have the authority to suspend or revoke a certificate of registration. The CCC is also authorized to conduct a secret shopper program to ensure compliance with all applicable laws and regulations.
U.S. Attorney Statements in Massachusetts
In January 2018, Andrew E. Lelling, the US Attorney for the District of Massachusetts, issued the following statement: “I understand that there are people and groups looking for additional guidance from this office about its approach to enforcing federal laws criminalizing marijuana cultivation and trafficking. I cannot, however, provide assurances that certain categories of participants in the state-level marijuana trade will be immune from federal prosecution. This is a straightforward rule of law issue. Congress has unambiguously made it a federal crime to cultivate, distribute and/or possess marijuana. As a law enforcement officer in the Executive Branch, it is my sworn responsibility to enforce that law, guided by the Principles of Federal Prosecution. To do that, however, I must proceed on a case-by-case basis, assessing each matter according to those principles and deciding whether to use limited federal resources to pursue it. Deciding, in advance, to immunize a certain category of actors from federal prosecution would be to effectively amend the laws Congress has already passed, and that I will not do. The kind of categorical relief sought by those engaged in state-level marijuana legalization efforts can only come from the legislative process.”
Michigan
Michigan Medical Regulatory Landscape
In November 2008, Michigan residents approved the Michigan Medical Marihuana Act (the “MMMA”) to provide a legal framework for a safe and effective medical marijuana program. In September 2016, the Michigan Legislature passed the Medical Marihuana Facilities Licensing Act (the “MMFLA”) and the Marihuana Tracking Act (the “MTA”) to provide a comprehensive licensing and tracking scheme, respectively, for the medical marijuana program. On November 6, 2018, Michigan voters approved Proposal 1, to make marijuana legal under state and local law for adults 21 years of age or older and to control the commercial production and distribution of marijuana under a
-29-
system that licenses, regulates, and taxes the businesses involved. The act is known as the Michigan Regulation and Taxation of Marihuana Act (“MRTMA” and together with the MMMA, the MMFLA, and the MTA, the “Michigan Cannabis Regulations”). Additionally, the Michigan Marijuana Regulatory Agency (“MRA”) has supplemented the Michigan Cannabis Regulations with administrative rules to further clarify the regulatory landscape surrounding the cannabis industry in Michigan. MRA is the main regulatory authority for the licensing of marijuana businesses.
In 2016, the Michigan legislature passed two new acts and also amended the original MMMA. The first act, amended effective January 1, 2019, establishes a licensing and regulation framework for medical marijuana growers, processors, secure transporters, provisioning centers, and safety compliance facilities. The second act establishes a “seed-to-sale” system to track marijuana that is grown, processed, transferred, stored, or disposed of under the Medical Marihuana Facilities Licensing Act.
The MRA is responsible for the oversight of cannabis in Michigan and consists of the Licensing Division and the Michigan Medical Marihuana Program Division. The MMMA provides access to state residents to cannabis and cannabis related products under one of 11 debilitating conditions, including epilepsy, cancer, HIV, AIDS, cancer and PTSD. In July 2018, the Medical Marihuana Facility Licensing Division approved 11 additional conditions to the list of aliments to qualify for medical cannabis, including chronic pain, colitis and spinal cord injury.
Allowable forms of marijuana includes smokable dried flower, dried flower for vaporizing and marijuana-infused products, which are defined under the Act to include topical formulations, tinctures, beverages, edible substances or similar products containing usable marijuana that is intended for human consumption in a matter other than smoke inhalation. Under the Michigan Cannabis Regulations, marijuana-infused products shall not be considered food.
Michigan Licensing Requirements
Under the MMFLA, MRA administrates five types of “state operating licenses” for medical marijuana businesses: (a) a “grower” license, (b) a “processor” license, (c) a “secure transporter” license, (d) a “provisioning center” license and (e) a “safety compliance facility” license. There are no stated limits on the number of licenses that can be made available on a state level; however, MRA has discretion over the approval of applications and municipalities can pass additional restrictions.
According to the MRTMA, MRA was required to start accepting applications for adult use establishments within 12 months of the measure’s effective date, and did so beginning in November 2019. Under the MRTMA, MRA administrates six types of “state operating licenses” for adult use marijuana businesses: (a) a “grower” license, (b) a “processor” license, (c) a “secure transporter” license, (d) a “provisioning center” license (e) a “safety compliance facility” license, and (f) a “microbusiness” license. The MRTMA also allows MRA to create additional license types and, to date, it has created three additional license types: (a) a “designated consumption establishment” license, (b) a “marihuana event organizer” license, (c) a “temporary marihuana event” license, and (d) an “excess marihuana grower” licenses. There are no stated limits on the number of licenses that can be made available on a state level; however, MRA has discretion over the approval of applications and municipalities can pass additional restrictions.
PharmaCo currently holds the following licenses (as more fully described in “Regulatory Overview – Cannabis
Licenses Held by State and Investee”):
·Provisioning Centers: 8 medical municipal licenses, 5 adult use municipal licenses, 8 medical state licenses and 5 adult use state licenses;
·Cultivation: 8 medical municipal licenses, 1 adult use municipal license, 6 medical state licenses and 1 adult use state license for cultivation;
·Processing: 2 medical municipal licenses; and
·Events: 1 adult use state license.
-30-
To the Company’s knowledge, PharmaCo operates in full compliance with licensing requirements and state and local regulations for cannabis-related activities and has not received any unresolved notices of violations with respect to any of its cannabis-related activities by any regulatory authority. PharmaCo will promptly disclose to the Company any non-compliance, citations or notices of violation which may have an impact on its licenses, business activities or operations.
Michigan Recordkeeping and Reporting
Pursuant to the requirements of the MTA, Michigan selected Franwell Inc.’s Marijuana Enforcement Tracking Reporting Compliance (“METRC”) software as the state’s third-party solution for integrated marijuana industry verification. Using METRC, regulators are able to track third party inventory, permissible sales and seed-to-sale information. Additionally, provisioning centers can use the METRC API to connect their own inventory management and/or point-of-sale systems to verify the identity as well as permissible sales for Michigan Qualified Purchasers.
Michigan Storage and Security
To ensure the safety and security of cannabis business premises and to maintain adequate controls against the diversion, theft, and loss of cannabis or cannabis products, a provisioning center is required to maintain and submit a security operations plan that includes the following at a minimum:
·Escorts for all non-employee personnel in limited access areas;
·Secure locks for all interior rooms, windows and points of entry and exits with commercial grade, non- residential door locks;
·An alarm system, with all information related to such alarm system including monitoring and alarm activity available to MRA;
·A video surveillance system that, at a minimum, consists of digital or network video recorders, cameras, video monitors, digital archiving devices and a color printer capable of delivering still photos;
·24-hour surveillance footage with fixed, mounted cameras, tamper/theft proof secured storage mediums and a notification system for interruption or failure of surveillance footage or storage of surveillance footage. All surveillance footage must be of sufficient resolution to identify individuals, have accurate time/date stamps and be stored for a minimum of 14 days unless state regulators notify that such recordings may be destroyed. Surveillance footage must cover:
oAll activity within 20 feet of all points of entry and exit to a facility;
oAny areas where marijuana products are weighed, packed, stored loaded, and unloaded for transportation, prepared or moved within the marijuana facility;
oLimited-access areas and security rooms, transfers between rooms;
oAreas storing a surveillance system storage device with at least 1 camera recording the access points to the secured surveillance recording area;
oAll entrances and exists to the building must be recorded from both indoor and outdoor vantage points, the areas of entrance and exit between marijuana facilities at the same location if applicable, including any transfers between marijuana facilities;
oPoint of sale areas where Michigan Marijuana products are sold and displayed for sale;
oState access to view and obtain copies of any surveillance footage through MRA or related investigators, agents, auditors and/or state police. A facility shall also provide copies of recordings to MRA upon request;
-31-
·Logs of the following: the identities of the employee or employees responsible for monitoring the video surveillance system, the identity of the employee who removed the recording from the video surveillance system storage device and the time and date removed, and the identity of the employee who destroyed any recording.
Maintain marijuana storage plan for provisioning centers that includes at a minimum:
* A secured limited access area for inventories of Michigan Marijuana Products;
* Clearly labeled containers that are:
oMarked, labeled or tagged;
oEnclosed on all sides;
oLatched or locked to keep all contents secured within. All such containers must be identified and tracked in accordance with the MTA;
* A locked area for chemical and solvents separate from Michigan Marijuana Products;
* Separation of marijuana-infused products from toxic or flammable materials;
* A sales or transfer counter or barrier separated from stock rooms to ensure registered qualifying patients or registered primary caregivers do not have direct access to Michigan Marijuana Products.
Michigan Dispensary Requirements
Michigan marijuana products may be purchased in a retail setting from a provisioning center by a Michigan Qualified Purchaser that presents a valid registry identification card issued by MRA (a “Michigan Registry ID”). For a Michigan Qualified Purchaser to receive marijuana products, provisioning centers must deploy an inventory control and tracking system that is capable of interfacing with the statewide monitoring system to determine (a) whether a Michigan Qualified Purchaser holds a Michigan Registry ID and (b) whether the sale or transfer will exceed the then-current daily and monthly purchasing limit for the holder of the Michigan Registry ID.
Michigan marijuana products may also be purchased by an adult 21 years of age or older at an adult use retailer, so long as the individual provides sufficient identification indicating their age. For an individual to receive marijuana products, adult use retailers must deploy an inventory control and tracking system that is capable of interfacing with the statewide monitoring system to determine whether the sale or transfer will exceed the then-current daily and monthly purchasing limit for the individual.
U.S. Attorney Statements in Michigan
On November 8, 2018, United States Attorneys Matthew Schneider and Andrew Birge for the Eastern and Western Districts of Michigan, respectively, issued a joint statement regarding the legalization of adult-use marijuana in Michigan. They stated that since they had taken oaths to protect and defend the Constitution and the laws of the United States, they would not immunize the residents of Michigan from federal law enforcement. They stated that they would continue to the investigation and prosecution of marijuana crimes as they do with any other crime. They stated they would consider the federal law enforcement priorities set by the DOJ, the seriousness of the crime, the deterrent effect of prosecution, and the cumulative impact of the crime on a community, while also considering their ability to prosecute with limited resources. They stated that combating illegal drugs was just one of many priorities, and that even within the area of drugs, they were focused on combating the opioid epidemic. They stated that they have not focused on prosecution of low-level offenders, which they stated would not change (unless aggravating factors were present). They did state that certain crimes involving marijuana could pose serious risks and harm to a community, including interstate trafficking, involvement of other illegal drugs or activity, persons with criminal records, presence of firearms or violence, criminal enterprises, gangs and cartels, bypassing local laws and regulations, potential for environmental contamination, risks to minors, and cultivation on federal property.
-32-
Oklahoma
Oklahoma Regulatory Landscape
In 2018, Oklahoma voters approved State Question 788, which was codified as Sections 420-426 of Title 63 of the Oklahoma Statutes and known as the Oklahoma Medical Marijuana and Patient Protection Plan. The new law allows individuals to receive a medical marijuana license if an Oklahoma Board certified physician recommends such in accordance with accepted standards that a reasonable and prudent physician would follow when recommending any medication. Such license holders are allowed to lawfully possess certain amounts of marijuana. If a medical marijuana license holder is homebound, than the Oklahoma State Department of Health (“OSHD”) is required to make a caregiver license available, which allows the caregiver to serve as the designee of the medical marijuana license holder and to have the same rights as the medical marijuana license holder.
The OSHD created the Oklahoma Medical Marijuana Authority (“OMMA”) to oversee the state’s medical marijuana program. Under the Oklahoma Medical Marijuana and Patient Protection Plan, the OSHD is required to issue four primary types of licenses: medical marijuana dispensary, commercial grower, processor, and marijuana transporter. Applications for dispensary, grower, and processor license types were required to be made available on the OSHD website no later than 30 days after the passage of State Question 788 and, upon receipt of an application, the OSHD has two weeks to review each application. The OSHD also issues a number of other types of licenses, including for marijuana research facilities, marijuana education facilities, marijuana waste disposal facilities, and marijuana testing laboratories.
Oklahoma Licensing Regime
A medical marijuana dispensary licensee is permitted to conduct retail sales of marijuana or marijuana derivatives only to medical marijuana license holders of their caregivers. A dispensary is prohibited from being located within 1,000 feet of any public or private school entrance.
A commercial grower licensee may sell marijuana to a licensed retailer or processor, but not directly to a medical marijuana license holder. Such sales to licensed retailers or processors are considered wholesale sales and are not subject to taxation. There is no limit on how much marijuana a licensed grower can cultivate.
A processor licensee may take marijuana plants and distill or process such plants into concentrates, edibles, and other forms for consumption. A licensed processor may sell its products to a licensed retailer or other licensed processor, but may not sell products directly to a medical marijuana license holder. Sales to a licensed retailer or other processor are considered wholesale sales and not subject to taxation. A processor license may also process cannabis into a concentrated form for medical marijuana license holders for a fee.
A medical marijuana transporter license allows an entity to transport, store, and distribute marijuana and marijuana products between licensed facilities. A transporter license will be issued to qualifying applicants for grower, processor, or dispensary licenses to transport marijuana or marijuana products on behalf of that licensee to other licensees. A transporter license may also be issued to an independent business.
The Company has limited, indirect cannabis activities in Oklahoma through RWB Platinum Vape, which sublicenses its brand to arm’s length entities. None of the Company, its subsidiaries or investees currently have any cannabis licenses or pending cannabis license applications in Oklahoma.
Oklahoma Licensing Requirements
All applicants for a dispensary license must be Oklahoma residents, and if an applicant is an entity, it must be owned by an Oklahoma state resident (defined as residency in Oklahoma for at least two years immediately preceding the application date or five years of continuous Oklahoma residency in the 25 years immediately preceding the application) and be registered to do business in the state. If a commercial grower or processor license applicant is an individual, the applicant must be an Oklahoma resident. Commercial grower or processor license applicants who are entities are not required to be owned by Oklahoma residents, but all members,
-33-
managers, and board members must be Oklahoma residents and out-of-state residents may not own more than 25% of the entity.
If an applicant meets relevant residency requirements, the OSHD must approve applications where: (i) the applicant is at least 25 years of age; (ii) the applying individual or entity is registered to conduct business in Oklahoma; (iii) the applicant is not a sheriff, deputy sheriff, police officer, prosecuting officer, officer or employee of OMMA, or officer or employee of a municipality in which the commercial licensee is located; (iv) the applicant has not had his or her authority to be a caregiver revoked; (v) the applicant discloses all ownership; (vi) the applicant pays the relevant application fee; (vii) the applicant has not failed to file required taxes; and (viii) the applicant or, if a corporation, any of its officers, directors, or stockholders, has not been convicted of a nonviolent felony within the past two years, a felony within the past five years, or be currently incarcerated.
Oklahoma Recordkeeping and Reporting
Dispensaries are required to complete a monthly sales report to the OSDH on the 15th of each following month. The report must provide the weight of marijuana purchased at wholesale, the weight of marijuana sold to medical marijuana license holders, and account for any waste. The report must also show the dispensary’s total sales in dollars, tax collected in dollars, and taxes due in dollars. A dispensary may be subject to penalty if a gross discrepancy exists and cannot be explained. Fraudulent reporting violations within a two-year time period result in an initial fine of $5,000 for a first violation and license revocation for a second violation.
A licensed grower is required to complete a monthly sales report to the OSDH on the 15th of each subsequent month. The report must provide the amount of marijuana harvested in pounds, the amount of drying or dried marijuana on hand, the amount of marijuana sold to dispensaries and processors in pounds, and the amount of waste in pounds. The report must also show total wholesale sales in dollars. A licensed grower may be subject to penalty if a gross discrepancy exists and cannot be explained. Fraudulent reporting violations within a two-year time period result in an initial fine of $5,000 for a first violation and license revocation for a second violation.
A licensed processor is required to complete a monthly sales report to the OSDH on the 15th of each subsequent month. The report must provide the amount of marijuana purchased in pounds, the amount of marijuana cooked or processed in pounds, the amount of waste in pounds, and the total wholesale sale in dollars. A licensed processor may be subject to penalty if a gross discrepancy exists and cannot be explained. Fraudulent reporting violations within a two-year time period result in an initial fine of $5,000 for a first violation and license revocation for a second violation.
All marijuana licensees must keep certain records for a period of at least seven years from the date of creation, including all business records; any documents related to the processing, preparation, and/or testing of marijuana; documentation of every instance in which medical marijuana was sold or otherwise transferred or purchased or otherwise obtained from another licensee; and any and all records relating to the disposal or destruction of marijuana or waste.
Oklahoma Security Requirements
All commercial licensees must implement appropriate security measures to deter and prevent the unauthorized entrance into areas containing marijuana and the theft and diversion of marijuana. All commercial licensees are responsible for the security of all marijuana items on the licensed premises or all marijuana items in their possession during transit.
Oklahoma Transportation
Qualified applicants for a dispensary, grower, or processor license will also be issued a marijuana transportation license at the time of their dispensary, grower, or processor approval. Independent businesses may also receive a transportation license. Such a license allows the licensee to transport marijuana between licensed facilities. All marijuana or marijuana products must be transported in a locked container that is shielded from public view, clearly labeled as “Medical Marijuana or Derivative,” and in a secured area of the vehicle that is not accessible to the driver during transit. All vehicles used to transport marijuana must be equipped with GPS trackers and properly
-34-
insured. All commercial transporters, growers, processors, and dispensaries must maintain accurate records and information on all vehicles engaged in the transport of marijuana. Agents of the transporter must keep a copy of the license on hand while transporting marijuana. Transporters must abide with all applicable motor vehicle laws and implement appropriate security measures to deter and prevent the theft and diversion of marijuana during transport.
Only agents, employees, officers, or owners of commercial transporters, growers, processors, or dispensaries who are issued a transporter agent license shall be qualified to transport marijuana. Commercial transporters, growers, processors, and dispensaries must utilize an electronic inventory management system to create and maintain shipping manifests documenting all transport of marijuana. A copy of such manifest shall be provided to each originating and receiving licensee at the time the product changes hands.
Oklahoma Inspections
Submission of an application for a commercial license constitutes permission for entry to and inspection of any licensed premises and any vehicle on the licensed premises used for the transportation of marijuana during hours of operation and other reasonable times. The OSHD may perform two on-site inspections per calendar year of each licensed grower, processor, dispensary, or commercial transporter to determine compliance with applicable law and rules. The OSHD must complete one on-site inspection of a testing laboratory applicant prior to initial licensure and one on-site inspection annually thereafter. The OSHD may conduct additional inspections to ensure correction of or investigate violations of applicable Oklahoma law and rules. The OSHD may review any and all records of a licensee and may require and conduct interviews with such persons or entities and persons affiliated with such entities, for the purpose of determining compliance with applicable laws and rules.
Cannabis Licenses Held by State and Investee
The below table describes the cannabis licenses held by Company’s subsidiaries and material investees, as well as information about state licenses currently outstanding and maximum licenses by state.
The Company’s subsidiaries and investees, to the Company’s knowledge, are compliant with all applicable state and local laws. The Company works with legal counsel to ensure that its subsidiaries with involvement in the U.S. legal cannabis industry maintain rigorous compliance programs, including developing, implementing and maintaining compliance programs and personnel training to ensure that operations do not endanger the health and safety of personnel or the communities in which the subsidiaries operate. The Company’s investees operate at arm’s length and, to the Company’s knowledge, maintain robust compliance and training programs to ensure full compliance with applicable cannabis laws.
The Company retains regulatory counsel in each jurisdiction in which it operates to ensure ongoing compliance and awareness of regulatory developments to continually enhance its compliance program. When evaluating new acquisition and investment opportunities, the Company engages proficient local counsel in a prospective new jurisdiction to ensure that any target is operating in compliance with all applicable state and local cannabis laws.
California
|
| Holder
| Relationship
| License Type
| License
| Approval
| Expiry Date
|
to Company
| Number
| Date
|
Vista Prime
| Subsidiary of
| Medicinal
| C11-
| 7/5/2019
| 7/4/2021
|
Management
| RWB
| Distributor
| 0000680-
|
LLC(1)
| Platinum
| Provisional
| LIC
|
| Vape
| License
|
|
Vista Prime
| Subsidiary of
| Annual
| CDPH-
| 3/28/2020
| 3/8/2021
|
Management
| RWB
| Manufacturing
| 10002204
|
LLC(1)
| Platinum
| License (Adult
|
|
| Vape
| and medicinal)
|
|
|
| Type 6: non-
|
|
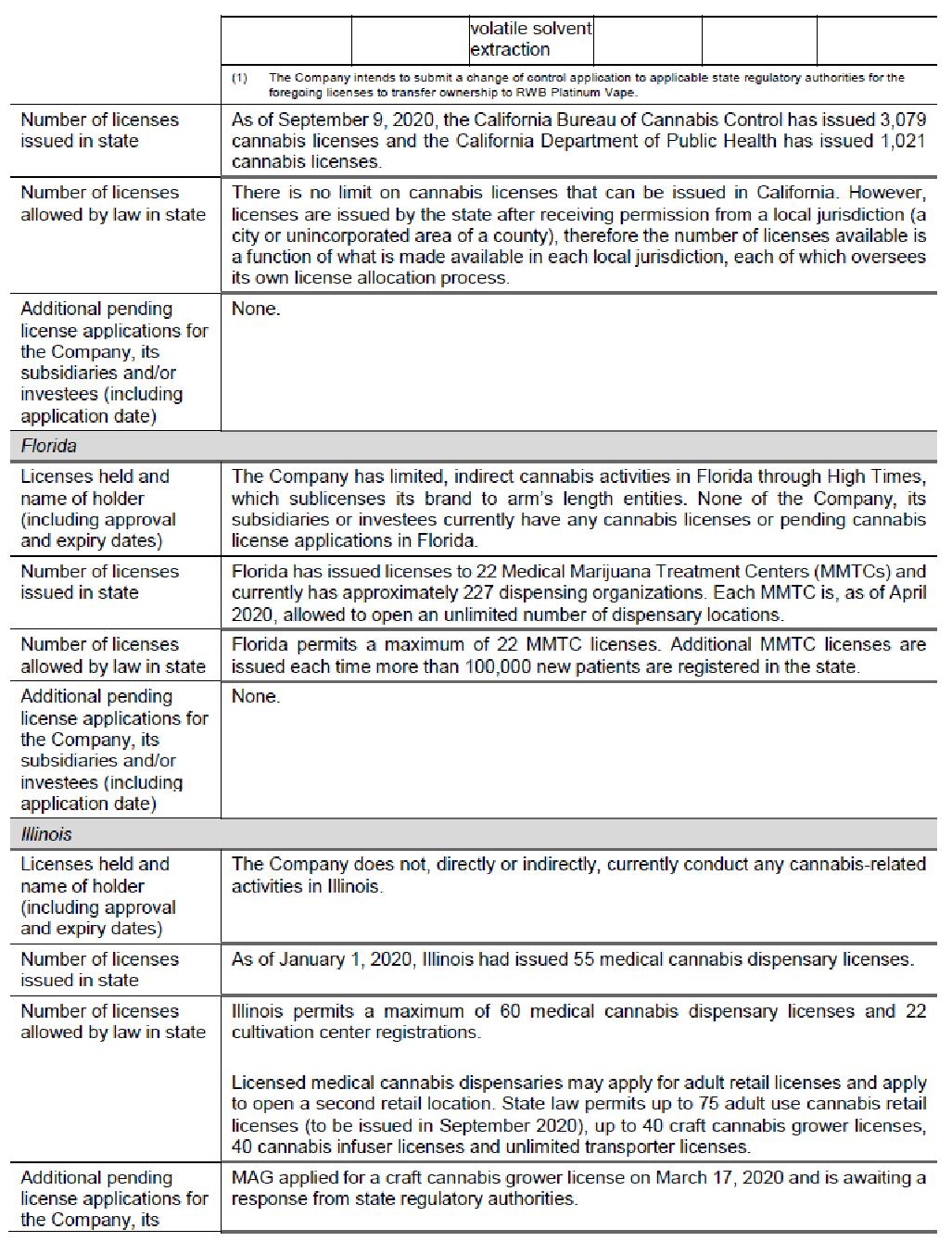
-36-
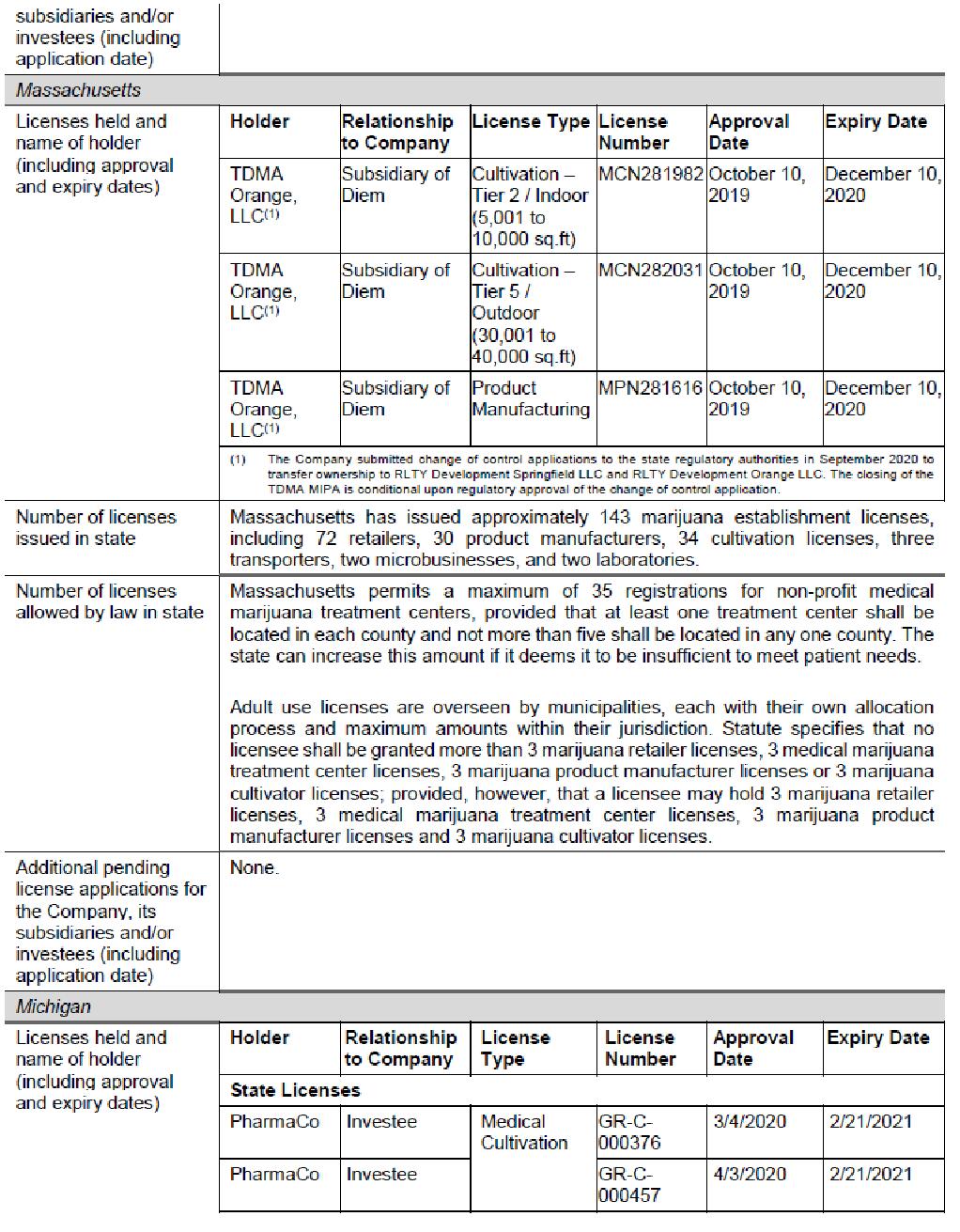
-37-
PharmaCo
| Investee
|
| GR-C-
| 4/3/2020
| 2/21/2021
|
| 000463
|
PharmaCo
| Investee
|
| GR-C-
| 4/3/2020
| 2/21/2021
|
| 000464
|
PharmaCo
| Investee
|
| GR-C-
| 4/3/2020
| 2/21/2021
|
| 000465
|
PharmaCo
| Investee
|
| GR-C-
| 5/2/2019
| 2/21/2021
|
| 000286
|
PharmaCo
| Investee
| Adult Use
| AU-G-C-
| 1/28/2020
| 12/17/2020
|
Cultivation
| 000122
|
PharmaCo
| Investee
| Medical
| PC-000249
| 3/21/2019
| 2/21/2021
|
PharmaCo
| Investee
| Provisioning
| PC-000261
| 4/25/2019
| 2/21/2021
|
Center
|
PharmaCo
| Investee
|
| PC-000232
| 2/21/2019
| 2/21/2021
|
PharmaCo
| Investee
|
| PC-000259
| 4/25/2019
| 2/21/2021
|
PharmaCo
| Investee
|
| PC-000226
| 3/21/2019
| 2/21/2021
|
PharmaCo
| Investee
|
| PC-000260
| 4/25/2019
| 2/21/2021
|
PharmaCo
| Investee
|
| PC-000267
| 6/24/2019
| 2/21/2021
|
PharmaCo
| Investee
|
| PC-000205
| 2/21/2019
| 2/21/2021
|
PharmaCo
| Investee
| Adult Use
| AU-R-
| 12/20/2019
| 12/17/2020
|
Provisioning
| 000130
|
PharmaCo
| Investee
| Center
| AU-R-
| 3/11/2020
| 12/17/2020
|
| 000193
|
PharmaCo
| Investee
|
| AU-R-
| 3/4/2020
| 12/17/2020
|
| 000194
|
PharmaCo
| Investee
|
| AU-R-
| 12/17/2019
| 12/17/2020
|
| 000143
|
PharmaCo
| Investee
|
| AU-R-
| 1/7/2020
| 12/17/2020
|
| 000145
|
PharmaCo
| Investee
| Adult Use
| AU-MEOA-
| 4/1/2020
| 4/1/2021
|
Event
| 000114
|
License
|
|
Municipal Licenses
|
PharmaCo
| Investee
| Medical
| BUS2020-
| 2/7/2020
| 2/7/2021
|
Cultivation
| 00030
|
PharmaCo
| Investee
|
| BUS2020-
| 2/7/2020
| 2/7/2021
|
| 00030
|
PharmaCo
| Investee
|
| BUS2020-
| 2/7/2020
| 2/7/2021
|
| 00030
|
PharmaCo
| Investee
|
| BUS2020-
| 2/7/2020
| 2/7/2021
|
| 00030
|
PharmaCo
| Investee
|
| BUS2020-
| 2/7/2020
| 2/7/2021
|
| 00030
|
PharmaCo
| Investee
|
| MMP20-
| 4/12/2019
| 6/30/2021
|
| 0184
|
-38-
| PharmaCo
| Investee
|
| MMP20-
| 2/26/2019
| 6/30/2021
|
| 0199
|
PharmaCo
| Investee
|
| ClsCGrower
| 8/13/2019
| 8/13/2021
|
| 2019-0003
|
PharmaCo
| Investee
| Adult Use
| RECP20-
| 12/10/2019
| 6/30/2021
|
Cultivation
| 0042
|
PharmaCo
| Investee
| Medical
| BUS2019-
| 3/14/2019
| 7/30/2021
|
Provisioning
| 00070
|
PharmaCo
| Investee
| Center
| BUS2019-
| 3/14/2019
| 7/30/2021
|
| 00068
|
PharmaCo
| Investee
|
| BUS2019-
| 3/14/2019
| 6/30/2021
|
| 00069
|
PharmaCo
| Investee
|
| MMP20-
| 3/21/2019
| 6/30/2021
|
| 0202
|
PharmaCo
| Investee
|
| MMP20-
| 6/11/2019
| 6/30/2021
|
| 0198
|
PharmaCo
| Investee
|
| 2020-01
| 6/30/2019
| 5/14/2021
|
PharmaCo
| Investee
|
| Letter of
| 4/5/2019
| 12/30/2020
|
| License-no
|
| number
|
PharmaCo
| Investee
|
| 2019-19
| 3/8/2019
| 12/12/2020
|
PharmaCo
| Investee
| Adult Use
| RECP20-
| 9/10/2019
| 6/30/2021
|
Provisioning
| 0038
|
PharmaCo
| Investee
| Center
| RECP20-
| 11/12/2019
| 6/30/2021
|
| 0039
|
PharmaCo
| Investee
|
| 2020-01
| 2/25/2020
| 2/24/2021
|
PharmaCo
| Investee
|
| Letter of
| 5/19/2020
| 12/31/2020
|
| License-no
|
| number
|
PharmaCo
| Investee
|
| 2019-3
| 11/11/2019
| 12/12/2020
|
PharmaCo
| Investee
| Medical
| Processor
| 8/30/2019
| 8/30/2021
|
Processing
| 2019-002
|
PharmaCo
| Investee
| Facility
| MMP20-
| 2/26/2019
| 6/30/2021
|
| 0200
|
Number of licenses
| As of the date of this prospectus, Michigan had issued 616 state cannabis licenses.
|
issued in state
|
Number of licenses
| There is no limit on medical or adult use licenses that can be issued in Michigan. However, a state and municipal license is required to operate and only certain municipalities have opted to allow cannabis businesses with local limits on permits and licenses. An individual can have an ownership interest in a maximum of five licensed adult use growers.
|
allowed by law in state
|
Additional pending
| None.
|
license applications for
the Company, its subsidiaries and/or investees (including application date)
|
-39-
Oklahoma
|
Licenses held and name of holder (including approval and expiry dates)
| The Company has limited, indirect cannabis activities in Oklahoma through RWB Platinum Vape, which sublicenses its brand to arm’s length entities. None of the Company, its subsidiaries or investees currently have any cannabis licenses or pending cannabis license applications in Oklahoma.
|
Number of licenses issued in state
| As of September 9, 2020, the state has issued 5,971 grower licenses, 1,328 processor licenses, 2,087 dispensary licenses, 39 transporter licenses, 23 laboratory licenses, and no waste disposal facility licenses.
|
Number of licenses allowed by law in state
| There is no limit on medical or adult use cannabis licenses that can be issued in
Oklahoma.
|
Additional pending license applications for the Company, its subsidiaries and/or investees (including application date)
| None.
|
CONSOLIDATED CAPITALIZATION
Change in Capitalization
The following summarizes the changes in the Company’s capitalization since January 31, 2020, the last day of the Company’s most recently completed fiscal period in respect of which financial statements have been filed, after giving effect to the Offering. The following table should be read in conjunction with the Annual Financial Statements and the Annual MD&A incorporated by reference in this Prospectus.
Description of Capital
| Outstanding as at
January 31, 2020(1)
| Outstanding as at
June 30, 2020 prior to giving effect to the Offering
| Outstanding as at
the date of this Prospectus after giving effect to the Offering (assuming the full exercise of the Over-Allotment Option and issuance of Compensation Options)
|
Share Capital
| Unlimited Common Shares; unlimited Series I Preferred Shares
| Unlimited Common Shares; unlimited Series I Preferred Shares; unlimited
Series II Preferred
Shares
| Unlimited Common Shares; unlimited Series I Preferred Shares; unlimited
Series II Preferred
Shares
|
Common Shares
| 304,572,662
| 151,421,886
| 187,272,886
|
Series I Preferred Shares
| 50,900,000
| 3,181,250
| 3,181,250
|
Series II Preferred Shares(2)
| Nil
| 112,040,549
| 112,540,549
|
Options
| 28,785,766
| 8,599,289(3)
| 8,099,289(5)
|
-40-
Warrants
| 113,806,365
| 595,340(4)
| 35,946,340(6)
|
MAG Milestone Share Rights
| Nil
| 2,640,000(7)
| 2,640,000(7)
|
Special Warrant
| Nil
| 4,500,000(8)
| 4,500,000(8)
|
Rights Under the PharmaCo
Put/Call Agreement
| Nil
| 37,000,000(9)
| 37,000,000(9)
|
Notes:
(1) The outstanding securities of the Company were consolidated on a 16:1 basis immediately prior to closing of the Business Combination
Transaction.
(2) The Series II Preferred Shares were created immediately prior to closing of the Business Combination Transaction. In connection with the
Business Combination Transaction, holders of MichiCann common shares received one Common Share and one Series II Preferred Share. (3) Exercisable for 8,599,289 Common Shares and 7,401,429 Series II Preferred Shares.
(4) Exercisable for 595,430 Common Shares and 595,340 Series II Preferred Shares.
(5) Exercisable for 8,099,289 Common Shares and 7,401,429 Series II Preferred Shares. (6) Exercisable for 35,946,340 Common Shares and 595,340 Series II Preferred Shares.
(7) Issuable pursuant to the Agreement and Plan of Merger dated as of January 9, 2020 as amended by and among MichiCann, MAG, RWB Illinois and Arthur VanWingerden and Ken VanWingerden upon the issuance of a commercial cultivation center license by the State of Illinois Department of Agriculture for MAG’s Illinois facility by December 31, 2021.
(8) Exercisable for 4,500,000 Common Shares.
(9) Exercisable for 37,000,000 Common Shares and 37,000,000 Series II Preferred Shares.
Debt Obligations
On February 25, 2019, the Company advanced $15,000,000 to MichiCann pursuant to a senior secured convertible debenture which was amended on August 28, 2019 pursuant to a first amending agreement (the “First Amending Agreement”), September 11, 2019 pursuant to a second amending agreement and March 12, 2020 pursuant to a third amending agreement (the “Third Amending Agreement”) (together, the “MichiCann Debenture”). The MichiCann Debenture is non-interest bearing, other than in the event of default by MichiCann. The MichiCann Debenture is secured by way of a security interest against the personal property of MichiCann which security interest is subordinated to the security interest held by Bridging. On August 28, 2019, the Company advanced MichiCann an additional US$2,000,000 pursuant to the First Amending Agreement and on March 12, 2020, an additional US$500,000 pursuant to the Third Amending Agreement to fund MichiCann working capital.
The MichiCann Debenture is secured against the assets of MichiCann pursuant to a general security and pledge agreement dated February 25, 2019 which security interests have been subordinated behind the security interest held by Bridging.
Following the completion of the Business Combination Transaction, the MichiCann Debenture remains as an intercorporate debt as between the Company and MichiCann.
On June 4, 2019, MichiCann and PharmaCo (collectively, the “Borrowers”) entered into a credit agreement (the “Credit Agreement”) with Bridging pursuant to which Bridging established a non-revolving credit facility (the “Facility”) for the Borrowers in a maximum principal amount of $36,374,400 (the “Facility Limit”).
The purpose of the Facility is so that PharmaCo can purchase certain real estate and business assets in the state of Michigan, to make additional permitted acquisitions and for general corporate and operating purposes.
The obligations under the Facility were due and payable on the earlier of: (a) the termination date (being January 4, 2020); and (b) the acceleration date (being the earlier of the date of an insolvency event or that a demand notice is delivered pursuant to the terms of the Credit Agreement). In respect of the advance made by Bridging to the Borrowers under the Facility, the Borrowers agreed to pay Bridging: Interest at the prime rate plus 10.55% per annum calculated and compounded monthly, payable monthly in arrears on the last day of each month; and a work fee equal to $909,360 (the “Work Fee”) was paid to Bridging.
The obligations under the Facility are secured by general security agreements on each Borrower, mortgages on certain owned real property of PharmaCo among other security obligations. As the funds under the Facility (net of
-41-
the Work Fee, commissions and other transaction expenses of Bridging) were advanced by Bridging directly to MichiCann, MichiCann in turn advanced the funds (net of MichiCann’s transaction expenses) to PharmaCo pursuant to a Promissory Note (the “Promissory Note”) issued by PharmaCo to MichiCann in the principal amount of $30,648,516.53 (the “Principal”). The Principal was due and payable in full on January 2, 2020 (the “Maturity Date”).
On January 10, 2020, the Facility was amended (the “Amended Facility”) pursuant to an amended and restated credit agreement between Bridging, MichiCann (as guarantor, instead of as borrower) and PharmaCo, RWB Illinois and MAG (as borrowers) (the “Amended Credit Agreement”). The Company has guaranteed the obligations of PharmaCo, RWB Illinois and MAG under the Amended Credit Agreement.
The Amended Facility increased the Facility Limit to US$49,750,000 in the aggregate of which US$27,000,000 was to refinance the existing Facility and US$22,750,000 was used by the Company to complete the acquisition of MAG and for general corporate and operating purposes. The obligations under the Amended Facility are now due and payable on the earlier of: (a) the termination date (being July 10, 2021 subject to the right of the Borrowers to extend the termination date by paying a 1% fee for two additional six-month periods for a total of 30 months); and (b) the acceleration date (being the earlier of the date of an insolvency event or that a demand notice is delivered pursuant to the terms of the Amended Credit Agreement). In respect of the advance made by Bridging to the Borrowers under the Facility, the Borrowers agreed to pay Bridging: Interest at the prime rate plus 12% per annum calculated and compounded monthly, payable monthly in arrears on the last day of each month. PharmaCo may prepay the Principal in full in whole prior to the Maturity Date. Any amounts payable by PharmaCo or MichiCann to Bridging under the Facility will reduce the amount of PharmaCo’s obligations to MichiCann on a dollar for dollar basis under the Promissory Note.
USE OF PROCEEDS Proceeds
The net proceeds to the Company from the Offering are estimated to be $20,445,000, after deducting the payment of the Underwriters’ Fee of $1,305,000, but before deducting the expenses of the Offering (estimated to be approximately $450,000 excluding taxes and disbursements). If the Over-Allotment Option is exercised in full for Over-Allotment Units, the net proceeds to the Company from the Offering are estimated to be $23,511,750, after deducting the Underwriters’ Fee of $1,500,750, but before deducting the expenses of the Offering.
Principal Purposes, Business Objectives and Milestones
The Company intends to use the net proceeds from the Offering as follows:
(a) to finance acquisition and investment activity over the next 4 to 6 months, including the repayment of certain amounts advanced by 1260356 Ontario Limited on September 9, 2020 pursuant to the Convertible Debenture and in connection with the initial payment under the Platinum Vape Definitive Agreement (such transactions are more fully described in the material change reports dated September 8, 2020 and September 15, 2020 and incorporated by reference into this Prospectus);
(b) to finance ongoing MAG operations over the next 4 months, including cultivation, processing, packaging and delivery of hemp products to market;
(c) to expand the Company’s executive team over the next 12 months with new members tasked with implementing brand expansion and optimal use of HT sublicensing rights. The Company will commence its search for additional executives in Q4 2020 and the Company’s compensation committee will determine remuneration;
(d) to finance ongoing regulatory matters over the next 12 months related to the procurement of cannabis licenses in jurisdictions in which the Company currently operates or is contemplating operations, including, but not limited to, approximately $400,000 allocated to ongoing matters in
-42-
Michigan in connection with the proposed acquisition of PharmaCo, $400,000 allocated to a cannabis license application in Illinois, $400,000 allocated to the change of control application for cannabis licenses in Massachusetts, $400,000 allocated to the change of control application for cannabis licenses in California held by subsidiaries of RWB Platinum Vape and $400,000 allocated to regulatory matters related to High Times brand sublicensing pursuant to the terms of the RWB Licensing Acquisition. See “Regulatory Overview”; and
(e) inventory, ongoing capital expenditures and general corporate purposes, including approximately
$995,000 allocated to purchasing packaging equipment, approximately $500,000 allocated to general maintenance and upkeep of existing facilities and equipment and approximately $500,000 allocated to general corporate purposes.
The use of the net proceeds from the Offering is consistent with the Company’s business objective of achieving growth through investing in and financing companies in the U.S. legal cannabis industry. At this time, the Company has not set specific milestones for the achievement of this objective. To the extent the Company requires additional capital to further the above purposes, it may raise funds through debt and equity financing in the future. See “Risk Factors – Risks Related to the Offering - Additional issuances and financings may result in dilution”.
The Company intends to allocate the net proceeds of the Offering for the following approximate purposes:
Purpose
| Net Proceeds of the Offering prior to
exercise of the Over-Allotment
Option(1)
($)
|
General and administrative, including estimated expenses and fees related to the Offering
| 450,000
|
Acquisition and investment activity
| 10,000,000
|
MAG operations
| 4,000,000
|
Expansion of executive team
| 2,000,000
|
Ongoing regulatory matters related to cannabis licenses
| 2,000,000
|
Inventory, ongoing capital expenditures and general corporate purposes
| 1,995,000
|
TOTAL
| $20,445,000
|
Notes:
(1) If the Over-Allotment Option is exercised in full, the excess net proceeds will be allocated to inventory, ongoing capital expenditures and general corporate purposes.
As at August 31, 2020, the Company’s working capital was approximately ($22,900,000), a change of $70,800,000 from the end of the most recent financial period on June 30, 2020. This is due primarily to the Company’s re- classification of the Amended Facility. With the termination date of the Amended Facility being July 10, 2021, the outstanding balance of the Amended Facility becomes a current liability instead of a long-term liability as of August 31, 2020 (subject to the Company exercising its right to extend the termination date. See “Consolidated Capitalization – Debt Obligations”). Without this re-classification, the Company’s working capital was reduced by $3,000,000, an amount in line with its current average spending. The Company intends to repay the Amended
-43-
Facility on its maturity date using cash flow from operations, through the refinancing of the Amended Facility or through a subsequent financing. None of the proceeds from the Offering will be used to repay the Amended Facility.
The Company’s intentions with respect to its use of proceeds are based on current knowledge, planning and expectations of management of the Company. Actual expenditures may differ from the expectations set forth above. There may be circumstances where for sound business reasons, the Company reallocates the use of proceeds, see “Risk Factors – Risks Related to the Offering – Discretion in the Use of Proceeds”. The Company had negative operating cash flow for the financial year ended July 31, 2019. The Company cannot guarantee that it will attain or maintain positive cash flow status in the future. To the extent that the Company has negative cash flow in any future period, certain of the proceeds from the Offering may be used to fund such negative cash flow from operating activities in these periods. See “Risk Factors – Risks Related to the Business – Cash flow from operations”.
Until applied, the net proceeds will be held as cash balances in the Company’s bank account or invested in certificates of deposit and other instruments issued by banks or obligations of or guaranteed by the Government of Canada or any province thereof in accordance with the Company’s investment policies. If the Over-Allotment Option is exercised in full for Over-Allotment Units, the Company will receive additional net proceeds of $3,066,750 after deducting the Underwriters’ Fee. The net proceeds from the exercise of the Over-Allotment Option, if any, is expected to be added to general working capital.
MARKET FOR SECURITIES Trading Price and Volume
RWB Common Shares
The Common Shares are currently traded on the CSE under the symbol “RWB”. The following table sets forth the reported price ranges and volume of trading for each month since August 2019. The Common Shares were halted on February 11, 2019 in connection with the Business Combination Transaction and resumed trading on June 5, 2020.
Period
| High
| Low
| Volume
|
September 1-17, 2020
| $0.74
| $0.57
| 5,416,798
|
August 2020
| $1.04
| $0.62
| 12,665,233
|
July 2020
| $1.30
| $0.82
| 14,428,694
|
June 2020
| $1.35
| $0.86
| 19,472,219
|
May 2020
| N/A
| N/A
| N/A
|
April 2020
| N/A
| N/A
| N/A
|
March 2020
| N/A
| N/A
| N/A
|
February 2020
| N/A
| N/A
| N/A
|
January 2020
| N/A
| N/A
| N/A
|
December 2019
| N/A
| N/A
| N/A
|
November 2019
| N/A
| N/A
| N/A
|
October 2019
| N/A
| N/A
| N/A
|
September 2019
| N/A
| N/A
| N/A
|
Prior Sales
The following table sets forth the details regarding all issuances of Common Shares, including issuances of all securities convertible or exchangeable into Common Shares, during the 12-month period before the date of this Prospectus:
-44-
Date of Issuance
| Number of
Securities Issued
| Type
| Issuance /
| Nature of Consideration
|
Exercise
Price Per Security
| Received
|
January 20, 2020
| 62,500
| Common Shares
| 0.80
| Warrant Exercise
|
January 27, 2020
| 12,500
| Common Shares
| 0.80
| Warrant Exercise
|
January 29, 2020
| 100,000
| Common Shares
| 0.80
| Warrant Exercise
|
February 3, 2020
| 187,500
| Common Shares
| 0.80
| Warrant Exercise
|
February 4, 2020
| 62,500
| Common Shares
| 0.80
| Warrant Exercise
|
February 5, 2020
| 25,000
| Common Shares
| 0.80
| Warrant Exercise
|
February 6, 2020
| 915,625
| Common Shares
| 0.80
| Warrant Exercise
|
February 7, 2020
| 859,813
| Common Shares
| 0.80
| Warrant Exercise
|
February 24, 2020
| 45,000
| Common Shares
| 0.80
| Warrant Exercise
|
February 25, 2020
| 4,375
| Common Shares
| 0.80
| Warrant Exercise
|
February 27, 2020
| 1,410,000
| Common Shares
| 0.80
| Warrant Exercise
|
February 28, 2020
| 843,250
| Common Shares
| 0.80
| Warrant Exercise
|
February 28, 2020
| 75,625
| Common Shares
| 0.80
| Warrant Exercise
|
April 24, 2020
| 108,726,349
| Common Shares
| N/A
| Business Combination
|
April 24, 2020
| 108,726,349
| Series II Preferred Shares
| N/A
| Business Combination
|
April 24, 2020
| 37,000,000
| Rights to acquire Common shares under PharmaCo Put/Call Agreement
| N/A
| Business Combination
|
April 24, 2020
| 37,000,000
| Rights to acquire Series II Preferred Shares under PharmaCo Put/Call Agreement
| N/A
| Business Combination
Transaction
|
April 24, 2020
| 2,640,000
| Milestone Common Shares issuable to MAG shareholders(1)
| N/A
| Business Combination
Transaction
|
April 24, 2020
| 2,640,000
| Milestone Series II Preferred Shares issuable to MAG shareholders(1)
| N/A
| Business Combination
Transaction
|
April 30, 2020
| 429,375
| Common Shares
| 0.80
| Warrant Exercise
|
May 25, 2020
| 187,500
| Common Shares
| 0.80
| Warrant Exercise
|
June 8, 2020
| 975,000
| Common Shares
| 0.25
| Stock Option Exercise
|
June 8, 2020
| 975,000
| Series II Preferred Shares
| 0.25
| Stock Option Exercise
|
June 10, 2020
| 15,300,000
| Common Shares
| 1.50
| RWB Licensing Acquisition
|
June 10, 2020
| 4,500,000
| Special Warrant exercisable into Common shares(2)
| N/A
| RWB Licensing Acquisition
|
June 10, 2020
| 1,800,000
| Common Shares(3)
| N/A
| RWB Licensing Acquisition
|
June 30, 2020
| 2,339,200
| Common Shares
| 1.25
| Debt Settlement re PharmaCo
|
June 30, 2020
| 2,339,200
| Series II Preferred Shares
| 1.25
| Debt Settlement re PharmaCo
|
August 13, 2020
| 500,000
| Common Shares
| 0.25
| Stock Option Exercise
|
August 13, 2020
| 500,000
| Series II Preferred Shares
| 0.25
| Stock Option Exercise
|
September 11, 2020
| 1
| Convertible Debenture(4)
| $10,000,000
| Convertible Debenture
|
Notes:
(1) Issuable pursuant to the Agreement and Plan of Merger dated as of January 9, 2020 as amended by and among MichiCann, MAG, RWB Illinois and Arthur VanWingerden and Ken VanWingerden upon the issuance of a commercial cultivation center license by the State of Illinois Department of Agriculture for MAG’s Illinois facility by December 31, 2021.
(2) Automatically exercisable into 4,500,000 Common Shares, if the volume weighted average price of the Common Shares on the CSE, for the first 180 days following June 10, 2020 is below $1.50.
(3) Issued to an arm’s length finder upon completion of the RWB Licensing Acquisition.
-45-
(4) The Convertible Debenture matures and is due and payable upon closing of the Offering. In the event the Offering is terminated, the Convertible
Debenture shall be convertible into such number of Units as is equal to the principal amount and accrued interest thereon.
DESCRIPTION OF SECURITIES BEING DISTRIBUTED
The following is a summary of certain general terms and provisions of the Unit Shares and Warrants offered and the Compensation Options to be granted pursuant to this Prospectus. This summary does not purport to be complete and is qualified in its entirety to the full text of the Warrant Indenture to be entered into on Closing.
This Prospectus qualifies the distribution of the Offered Units and the Compensation Options. Each Offered Unit consists of one Unit Share and one Warrant. The Unit Shares and the Warrants will separate immediately upon Closing.
Offering
The Offering consists of Offered Units offered at the Offering Price of $0.75 per Offered Unit. Each Offered Unit will consist of one Unit Share and one Warrant.
The Company’s Series I Preferred Shares and Series II Preferred Shares rank senior to the Company’s Common
Shares and are described below.
Common Shares
As at September 17, 2020, the Company had 151,921,886 Common Shares issued and outstanding.
The Common Shares of the Company entitle holders thereof to receive dividends as and when declared by the Board of Directors of the Company. In the event of liquidation, dissolution or winding-up of the Company, the holders of Common Shares are entitled to receive all the remaining property and assets of the Company. The holders of Common Shares are entitled to receive notice of and to attend and to vote at all meetings of the shareholders of the Company and each Common Share, when represented at any meeting of the shareholders of the Company, carries the right to one vote.
All of the Common Shares rank equally within their class as to dividends, voting rights, participation in assets and in all other respects. None of such Common Shares are subject to any call or assessment nor pre-emptive or conversion rights. Any modification, amendment or variation of any rights or other terms attached to the Common Shares would require special resolutions passed by the shareholders of the Company.
Common Shares issued to, or for the account or benefit of, persons in the United States or U.S. Persons, will be “restricted securities” within the meaning of Rule 144(a)(3) under the U.S. Securities Act. Certificates issued or book entries representing such securities will bear a legend or notation to the effect that the securities represented thereby are not registered under the U.S. Securities Act or any applicable U.S. state securities laws and may only be offered, sold, pledged or otherwise transferred pursuant to certain exemptions from the registration requirements of the U.S. Securities Act and any applicable U.S. state securities laws.
Series I Preferred Shares
The Company is authorized to issue an unlimited number of Series I Preferred Shares, which rank senior to the Common Shares. The terms of the Series I Preferred Shares provide, among other things, that they: (i) are non- voting; (ii) are convertible into Common Shares on a one for one basis, subject to customary adjustments; (iii) are eligible to participate in dividends if and when declared on the Common Shares; (iv) have priority rights on liquidation; and (v) are subject to a restriction that no holder of the Series I Preferred Shares may convert into a number of Common Shares that would result in such holder beneficially owning greater than 9.99% of the Company’s Common Shares.
-46-
Series II Preferred Shares
The Company is authorized to issue an unlimited number of Series II Preferred Shares, which rank senior to the Common Shares and the Series I Preferred Shares. Each Series II Preferred Share shall be convertible into one validly issued, fully paid and non-assessable Common Share on the terms and conditions set out in the Company’s constating documents. Each Series II Preferred Share is convertible into one Common Share any time after the date that is seven months after the closing date of the Business Combination Transaction and before the date that is twenty-four months after the closing date of the Business Combination Transaction.
Holders of Series II Preferred Shares shall be treated for all purposes as the record holder or holders of such underlying Common Shares on the date on which the Series II Preferred Shares are converted.
Holders of Series II Preferred Shares shall have voting rights and are entitled to vote on a matter with holders of Common Shares (and Series I Preferred Shares if required by law or otherwise entitled to vote with the holders of Common Shares), voting together as one class. Each Series II Preferred Share shall entitle the holder thereof to cast that number of votes per share as is equal to the number of Common Shares into which it is then convertible using the record date for determining the shareholders of the Company eligible to vote on such matters as the date as of which the Series II Preferred Share Conversion Rate (as defined in the Company’s constating documents) is calculated. Holders of the Series II Preferred Shares shall be entitled to written notice of all shareholder meetings or written consents (and copies of proxy materials and other information sent to shareholders), which notice shall be provided pursuant to the Company’s bylaws and applicable law.
Holders of Series II Preferred Shares shall be entitled to receive, and the Company shall pay thereon, a fixed dividend equal to 5.0% per annum, calculated monthly and payable in Series II Preferred Shares. Upon conversion of Series II Preferred Shares, the dividend shall be calculated pro rata as at the most recently completed month prior to the Series II Preferred Share Conversion Date (as defined in the Company’s constating documents). Holders of Series II Preferred Shares shall be entitled to receive such dividends paid and distributions made to the holders of the Common Shares to the same extent as if such Series II Preferred Share holders had converted each Series II Preferred Share held by them into Common Shares and had held such Common Shares on the record date for such dividends and distributions. Payment under the preceding sentence shall be made concurrently with the dividend or distribution to the holders of Common Shares. The Company will be entitled to deduct and withhold from any dividends paid in respect of Series II Preferred Shares, and to otherwise recover from the Series II Preferred Share holder the full amount of taxes or other additional amounts required to be deducted or withheld by the Company under applicable laws.
Warrants
Each Warrant will be transferable and will entitle the holder thereof to acquire one Warrant Share at a price of
$1.00 per Warrant Share at any time prior to 5:00 p.m. (EST) at any time up to 24 months following the Closing Date, subject to adjustment in certain customary events, after which time the Warrants will expire subject to an Accelerated Exercise Period.
The Warrants will be issued under and governed by the Warrant Indenture to be entered into on the Closing Date between the Company and National Securities Administrators Ltd., as warrant agent. The Company will appoint the principal transfer office of National Securities Administrators Ltd. in Vancouver as the location at which the Warrants may be surrendered for exercise, transfer or exchange. Under the Warrant Indenture, the Company may, subject to applicable law, purchase by private contract or otherwise, any of the Warrants then outstanding, and any Warrants so purchased will be cancelled.
The Warrant Indenture will provide for adjustment in the class and/or number of securities issuable upon the exercise of the Warrants and/or Exercise Price per Warrant Share in the event of the following additional events: (i) reclassifications of the Common Shares; (ii) consolidations, amalgamations, arrangements or mergers of the Company with or into any other corporation or other entity (other than consolidations, amalgamations, arrangements or mergers which do not result in any reclassification of the outstanding Common Shares or a change of the Common Shares into other shares); or (iii) the transfer of the undertaking or assets of the Company as an entirety or substantially as an entirety to another corporation or other entity.
-47-
No adjustment in the Exercise Price or the number of Warrant Shares issuable upon the exercise of the Warrants will be required to be made unless the cumulative effect of such adjustment or adjustments would result in a change of at least 1% in the Exercise Price or a change in the number of Warrant Shares issuable upon exercise by at least one one-hundredth of a Warrant Share, as the case may be.
The Company will covenant in the Warrant Indenture that, during the period in which the Warrants are exercisable, it will give notice to National Securities Administrators Ltd. and to the holders of the Warrants of certain stated events, including events that would result in an adjustment to the Exercise Price for the Warrants or the number of Warrant Shares issuable upon exercise of the Warrants, at least 14 days prior to the record date of such event, if any.
No fractional Warrant Shares will be issuable upon the exercise of any Warrants and no cash or other consideration will be paid in lieu of fractional Warrant Shares. Holders of Warrants will not have any voting, dividend or pre- emptive rights or any other rights which a holder of Common Shares would have.
The Warrant Indenture will provide that, from time to time, the Company may amend or supplement the Warrant Indenture for certain purposes, without the consent of the holders of the Warrants, including curing defects or inconsistencies or making any change that does not prejudice the rights of any holder. Any amendment or supplement to the Warrant Indenture that would prejudice the interests of the holders of Warrants may only be made by “extraordinary resolution”, which will be defined in the Warrant Indenture as a resolution either: (i) passed at a meeting of the holders of Warrants at which there are holders of Warrants present in person or represented by proxy holding at least 25% of the aggregate number of the then outstanding Warrants (unless such meeting is adjourned to a prescribed later date due to the lack of quorum) and passed by the affirmative vote of the holders of Warrants present in person or by proxy representing not less than 662/3% of the aggregate number of all the then outstanding Warrants represented at the meeting; or (ii) adopted by an instrument in writing signed by the holders of Warrants representing not less than 662/3% of the aggregate number of all the then outstanding Warrants.
The foregoing is a summary of all of the material provisions of the Warrant Indenture, it does not purport to be complete and is qualified in its entirety by reference to the provisions of the Warrant Indenture in the form to be agreed upon by the parties.
The Warrants may not be exercised in the United States, or by or for the account of a U.S. Person or a person in the United States except pursuant to exemptions from the registration requirements of the U.S. Securities Act and any applicable state securities laws, and the holder has delivered to the Company a written opinion of counsel, in form and substance satisfactory to the Company; provided, however, that a U.S. Purchaser that purchased the Warrants from the Company pursuant to Rule 144A or Rule 506(b) under Regulation D of the U.S. Securities Act for its own account, or for the account of another U.S. Purchaser for which it exercised sole investment discretion with respect to such original purchase (a “Original Beneficial Purchaser”) will not be required to deliver an opinion of counsel if it exercises the Warrants for its own account or for the account of the Original Beneficial Purchaser, if any, if each of it and such Original Beneficial Purchaser, if any, was either an Institutional Accredited Investor or Qualified Institutional Buyer, as applicable, at the time of its purchase and exercise of the Warrants.
Warrant Shares
Upon exercise of the Warrants, holders of the Warrant Shares shall be entitled, as holders of Common Shares, to all rights as a shareholder of the Company including the right to receive notice of and to attend all meetings of the shareholders of the Company and have one vote for each Common Share held at all meetings of the shareholders of the Company.
Warrant Shares issuable upon exercise of the Warrants to, or for the account or benefit of, persons in the United States or U.S. Persons, will be “restricted securities” within the meaning of Rule 144(a)(3) under the U.S. Securities Act. Certificates issued or book entries representing such securities will bear a legend or notation to the effect that the securities represented thereby are not registered under the U.S. Securities Act or any applicable U.S. state securities laws and may only be offered, sold, pledged or otherwise transferred pursuant to certain exemptions from the registration requirements of the U.S. Securities Act and any applicable U.S. state securities laws.
-48-
Compensation Options
As additional consideration for the services rendered in connection with the Offering, the Company has agreed to issue to the Underwriters such number of non-transferable Compensation Options to acquire that number of units of the Company on the same terms as the Offered Units as is equal to 6.0% of the number of Offered Units sold under the Offering (including upon the exercise of the Over-Allotment Option). Each Compensation Option shall be exercisable into one Compensation Unit consisting of one Compensation Share and one Compensation Warrant at the Offering Price for a period of 24 months following the Closing Date, subject to adjustment in certain customary events. Each Compensation Warrant will entitle the holder thereof to acquire one Compensation Warrant Share at the Exercise Price for a period of twenty-four (24) months following the Closing Date, subject to acceleration on the same terms as the Warrants. The Underwriters, as holders of the Compensation Options, will not as such have any voting right or other right attached to Common Shares until and unless the Compensation Options are duly exercised as provided for in the certificates representing the Compensation Options.
PLAN OF DISTRIBUTION General
This Prospectus qualifies the distribution of the Offered Units in each of the provinces of Canada other than Québec, to purchasers upon completion of the Offering and any Over-Allotment Units issued pursuant to the exercise of the Over-Allotment Option.
Pursuant to the Underwriting Agreement, the Company has agreed to sell and the Underwriters have severally (and not jointly or jointly and severally) agreed to purchase, as principals, on the Closing Date, 29,000,000 Offered Units at the Offering Price, for aggregate gross consideration of $21,750,000 payable in cash to the Company against delivery of the Offered Units. The obligations of the Underwriters under the Underwriting Agreement are several (and not joint or joint and several), are subject to certain closing conditions and may be terminated at their discretion on the basis of “disaster out”, “due diligence out”, “material adverse change out”, “restrictions on distributions out”, “breach of agreement out” and “regulatory proceedings out” provisions in the Underwriting Agreement and may also be terminated upon the occurrence of certain other stated events. The Underwriters are, however, obligated to take-up and pay for all of the Offered Units if any Offered Units are purchased under the Underwriting Agreement. The Offering Price was determined by arm’s-length negotiation between the Company and the Co-Lead Underwriter, on behalf of the Co-Lead Underwriters, with reference to the prevailing market price of the Common Shares.
The Underwriters have been granted an over-allotment option exercisable in whole or in part, at the sole discretion of the Underwriters by giving notice to the Company at any time, and from time to time, on or before 5:00 p.m. (EDT) on the 30th day following the Closing Date, to purchase up to an additional 15% of the number of Offered Units sold under the Offering, being up to 4,350,000 Over-Allotment Units and/or up to 4,350,000 Over-Allotment Unit Shares and/or up to 4,350,000 Over-Allotment Warrants, to cover the Underwriters’ Over-Allotment Option. The Over-Allotment Option may be exercised by the Underwriters to acquire: (a) Over-Allotment Units at the Offering Price; (b) Over-Allotment Unit Shares at a price of $0.67 per Over-Allotment Unit Share; (c) Over-Allotment Warrants at a price of $0.08 per Over-Allotment Warrant; or (d) any combination of Over-Allotment Units, Over- Allotment Unit Shares and Over-Allotment Warrants, so long as the aggregate number of Over-Allotment Unit Shares and Over-Allotment Warrants which may be issued under the Over-Allotment Option does not exceed 4,350,000 Over-Allotment Unit Shares and 4,350,000 Over-Allotment Warrants.
Upon completion of the Offering, the aggregate Underwriters’ Fees payable by the Company in respect of the Offering will be $1,305,000 ($1,500,750 if the Over-Allotment Option is exercised in full). The net proceeds to the Company, before deducting the expenses of the Offering, will be $20,445,000 ($23,511,750 if the Over-Allotment Option is exercised in full). The total number of Offered Units sold pursuant to the Offering will be 29,000,000 (33,350,000 if the Over-Allotment Option is exercised in full), and the total price to the public will be $21,750,000 ($25,012,500 if the Over-Allotment Option is exercised in full). As additional consideration for the services rendered in connection with the Offering, the Company has agreed to issue to the Underwriters such number of non- transferable Compensation Options to acquire that number of Compensation Units as is equal to 6.0% of the number of Offered Units sold under the Offering (including upon the exercise of the Over-Allotment Option). Each
-49-
Compensation Option will entitle the holder thereof to acquire one Compensation Unit consisting of one Compensation Share and one Compensation Warrant at an exercise price equal to the Offering Price for a period of 24 months following the Closing Date, subject to adjustment in certain customary events. Each Compensation Warrant will entitle the holder thereof to acquire one Compensation Warrant Share at the Exercise Price for a period of twenty-four (24) months following the Closing Date, subject to acceleration on the same terms as the Warrants. The Compensation Option Units will have the same terms as the Offered Units. This Prospectus qualifies the distribution of the Compensation Options to the Underwriters.
The expenses of the Offering, including the reasonable fees and disbursements and taxes thereon of the Underwriters’ counsel but not including the Underwriters’ Fees, are estimated to be $450,000 (excluding taxes and disbursements) and are payable by the Company.
Pursuant to the terms of the Underwriting Agreement, the Company has agreed to reimburse the Underwriters for certain expenses incurred in connection with the Offering and to indemnify the Underwriters and their directors, officers, employees, and agents against certain liabilities, including liabilities under securities legislation, and expenses and to contribute to payments the Underwriters may be required to make in respect thereof.
The Company has agreed, that until the date which is 90 days after the Closing Date, it will not, without the written consent of the Co-Lead Underwriters, after discussion therewith, which consent shall not be unreasonably withheld, directly or indirectly offer, issue, pledge, sell, contract to sell, announce an intention to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise lend, transfer or dispose of, directly or indirectly, any Common Shares or securities convertible into or exchangeable for Common Shares, other than: (i) the exercise of the Over-Allotment Option; (ii) the issuance of Common Shares in connection with the exercise of any currently outstanding options or warrants or other securities convertible into Common Shares of the Company, (iii) the issuance of options to acquire Common Shares pursuant to the Company’s stock option plan or other compensation arrangements in place prior to the date hereof; and (iv) to satisfy any other currently outstanding instruments or other contractual commitments in relation to any transaction that has been previously disclosed in writing to the Underwriters. The Company has also agreed to cause each of its directors and officers of the Company (as constituted on the date of the final Prospectus) to enter into an agreement in favour of the Underwriters pursuant to which each of such individuals will agree not to sell, transfer or pledge, or otherwise dispose of, any securities of the Company until the date which is 90 days after the date of the Closing, in each case without the prior written consent of the Underwriters, such consent not to be unreasonably withheld or delayed, subject to customary exceptions.
Subscriptions for the Offered Units will be received, subject to rejection or allotment in whole or in part, and the right is reserved to close the subscription books at any time without notice. Closing of the Offering is expected to take place on or about September 24, 2020, or such other date as may be agreed upon by the Company and the Co-Lead Underwriters, but in any event no later than 42 days after the date of the receipt for the (final) short form prospectus. It is anticipated that the Offered Units will be delivered under the book-based system through CDS or its nominee and deposited in electronic form. A purchaser of Offered Units will receive only a customer confirmation from the registered dealer from or through which the Offered Units are purchased and who is a CDS depository service participant. CDS will record the CDS participants who hold Units on behalf of owners who have purchased Offered Units in accordance with the book-based system. No certificates will be issued unless specifically requested or required.
The Company has applied to list the Unit Shares, the Warrants and the Warrant Shares (including those underlying the Over-Allotment Units and the Compensation Units) and the Compensation Shares, the Compensation Warrants and the Compensation Warrant Shares on the CSE. Listing will be subject to the Company fulfilling all listing requirements of the CSE.
The Offering is being made in each of the provinces of Canada except Québec. The Offered Units will be offered in each of the relevant provinces of Canada through the Underwriters or their affiliates who are registered to offer the Offered Units for sale in such provinces and such other registered dealers as may be designated by the Underwriters. Subject to applicable law, the Underwriters may offer the Offered Units in such other jurisdictions outside of Canada and the United States as agreed between the Company and the Co-Lead Underwriters.
-50-
The Underwriters propose to offer the Offered Units initially at the Offering Price. After the Underwriters have made a reasonable effort to sell all of the Offered Units at the Offering Price, the Offering Price may be decreased and may be further changed from time to time to an amount not greater than the Offering Price, and the compensation realized by the Underwriters will be decreased by the amount that the aggregate price paid by purchasers for the Offered Units is less than the gross proceeds paid by the Underwriters to the Company.
There is currently no market through which the Warrants may be sold and purchasers may not be able to resell the Warrants purchased under this Prospectus. See “Risk Factors – Risks Related to the Offering – Market for Warrants”.
United States Sales
The Offered Units, Unit Shares, Warrants and Warrant Shares have not been and will not be registered under the U.S. Securities Act or any state securities laws and may not be offered, sold or delivered, directly or indirectly, to, or for the account or benefit of, a person in the United States or a U.S. Person, unless pursuant to an exemption to the registration requirements of such laws. Accordingly, the Underwriters have agreed that it will not offer, sell or deliver the Offered Units, Unit Shares, Warrants or Warrant Shares within the United States except in certain transactions exempt from the registration requirements of the U.S. Securities Act and applicable state securities laws. The Underwriting Agreement will permit the Underwriters to (i) acting through its United States broker-dealer affiliate, to offer the Offered Units for sale by the Company in the United States and to or for the account or benefit of U.S. Persons to purchasers that are U.S. Accredited Investors, in compliance with Rule 506(b) of Regulation D under the U.S. Securities Act and applicable U.S. state securities laws, and (ii) offer and resell, acting through their United States broker-dealer affiliates, the Offered Units that they have acquired pursuant to the Underwriting Agreement in the United States and to, or for the account or benefit of U.S. Persons, that are Qualified Institutional Buyers in compliance with Rule 144A under the U.S. Securities Act and applicable U.S. state securities laws. In addition, the Underwriters will offer and sell the Offered Units outside the United States to non-U.S. Persons only in accordance with Regulation S under the U.S. Securities Act. The Unit Shares and Warrants comprising the Offered Units, and the Warrant Shares issuable upon exercise of the Warrants, as applicable, in each instance issued to, or for the account or benefit of, persons in the United States or U.S. Persons, will be “restricted securities” within the meaning of Rule 144(a)(3) under the U.S. Securities Act. Certificates issued or book entries representing such securities will bear a legend or notation to the effect that the securities represented thereby are not registered under the U.S. Securities Act or any applicable U.S. state securities laws and may only be offered, sold, pledged or otherwise transferred pursuant to certain exemptions from the registration requirements of the U.S. Securities Act and any applicable U.S. state securities laws.
This Prospectus does not constitute an offer to sell, or a solicitation of an offer to buy, any securities to, or for the account or benefit of, a person in the United States or a U.S. Person. In addition, until 40 days after commencement of the Offering, an offer or sale of the Offered Units, Unit Shares and Warrants within the United States by any dealer (whether or not participating in the Offering) may violate the registration provisions of the U.S. Securities Act unless such offer is made pursuant to an exemption from registration under the U.S. Securities Act and similar exemptions under applicable state securities laws.
Price Stabilization and Passive Market-Making
In connection with the Offering and subject to applicable laws, the Underwriters may over-allot or effect transactions that stabilize or maintain the market price of the Unit Shares or the Warrants, as applicable, at a level other than that which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time. The Underwriters may carry out these transactions on the CSE, in the over-the-counter market or otherwise.
Pursuant to policy statements of certain securities regulators, the Underwriters may not, throughout the period of distribution, bid for or purchase Common Shares. The foregoing restriction is subject to certain exceptions including: (i) a bid or purchase permitted under the Universal Market Integrity Rules for Canadian Marketplaces administered by the Investment Industry Regulatory Organization of Canada relating to market stabilization and passive market making activities, (ii) a bid or purchase made for and on behalf of a customer where the order was not solicited
-51-
during the period of the distribution, provided that the bid or purchase was for the purpose of maintaining a fair and orderly market and not engaged in for the purpose of creating actual or apparent active trading in, or raising the price of, such securities, or (iii) a bid or purchase to cover a short position entered into prior to the commencement of a prescribed restricted period.
As a result of these activities, the price of the Unit Shares may be higher than the price that otherwise might exist in the open market. If these activities are commenced, they may be discontinued by the Underwriters at any time. The Underwriters may carry out these transactions on any stock exchange on which the Common Shares are listed, in the over-the-counter market, or as otherwise permitted by applicable law.
RELATIONSHIP BETWEEN THE COMPANY AND CERTAIN UNDERWRITERS
A controlling shareholder of PI Financial Corp. is concurrently an influential shareholder of Bridging, which is a lender to certain subsidiaries of the Company (being MAG and RWB Illinois) pursuant to the Amended Facility under which such subsidiaries are currently indebted and which the Company has guaranteed. Consequently, the Company may be considered a “connected issuer” to PI Financial Corp. under applicable Canadian securities legislation. As at September 17, 2020, $65,490,910, was outstanding under the Amended Facility. Obligations under the Amended Facility are secured by general security agreements on each Borrower, mortgages on certain owned real property of PharmaCo among other security obligations.
As of the date hereof, the Company is in compliance, in all material respects, with the terms and conditions of all the agreements which govern its agreements with Bridging, and the Company has not received notice of any breach of any such terms or conditions.
The decision to distribute the Offered Units pursuant to the Offering and the determination of the terms of the Offering were made through arm’s length negotiation between the Company and the Co-Lead Underwriters. Bridging did not have any involvement in such decision or determination and the Offering was not otherwise required by, suggested by or subject to the consent of Bridging. Bridging will not receive any benefits in connection with the Offering. The Company does not anticipate any proceeds from the Offering being applied against the outstanding credit facilities noted above. See “Use of Proceeds”.
RISK FACTORS
An investment in the Offered Units is speculative and involves certain risks. When evaluating the Company and its business, prospective purchasers of the Offered Units should consider carefully the information set out in this Prospectus, including the documents incorporated by reference herein, and the risks described below and in the documents incorporated by reference herein, including those risks identified and discussed under the heading “Risk Factors” in the Annual Information Form, which is incorporated by reference herein. There is no assurance that risk management steps taken will avoid future loss due to the occurrence of the risks described below (or incorporated by reference herein) or other unforeseen risks. If any of the risks described below or in the Annual Information Form actually occur, then the Company’s business, prospects, financial condition and operating results could be materially adversely affected with the result that the trading price of the Common Shares could decline and investors could lose all or part of their investment.
The risks and uncertainties described or incorporated by reference herein are not the only ones the Company faces. Additional risks and uncertainties, including those that the Company is unaware of or that are currently deemed immaterial, may also adversely affect the Company and its business and prospects. The Company cannot provide any assurances that it will successfully address any or all of these risks. Investors should consult with their professional advisors to assess any investment in securities of the Company.
Risks Related to the Offering
Completion of the Offering
The completion of the Offering remains subject to a number of conditions. There can be no certainty that the Offering will be completed. Failure by the Company to satisfy all of the conditions precedent to the Offering would
-52-
result in the Offering not being completed. If the Offering is not completed, the Company may not be able to raise the funds required for the purposes contemplated under “Use of Proceeds” from other sources on commercially reasonable terms or at all.
Discretion in the Use of Proceeds
Management will have discretion concerning the use of the proceeds of the Offering as well as the timing of their expenditure. As a result, purchasers will be relying on the judgment of management for the application of the proceeds of the Offering. Management may use the net proceeds of the Offering other than as described under the heading “Use of Proceeds” if they believe it would be in the Company’s best interest to do so and in ways that a purchaser may not consider desirable. The results and the effectiveness of the application of the proceeds are uncertain. If the proceeds are not applied effectively, the Company results of operations may suffer.
Additional Financing
The continued development and investment objectives of the Company will require additional financing. There is no guarantee that the Company will be able to achieve its business objectives. The Company expects to fund its business objectives by way of additional offerings of equity and/or debt financing. The failure to raise or procure such additional funds could result in the delay or indefinite postponement of current business objectives. There can be no assurance that additional capital or other types of financing will be available if needed or that, if available, will be on terms acceptable to the Company. If additional funds are raised by offering equity securities or convertible debt, existing shareholders could suffer significant dilution. Any debt financing secured in the future could involve the granting of security against assets of the Company and also contain restrictive covenants relating to capital raising activities and other financial and operational matters, which may make it more difficult for the Company to obtain additional capital and to pursue business opportunities, including potential acquisitions. The Company may require additional financing to fund its operations and investment objectives. See “Additional Issuances and Financings May Result in Dilution”.
Market for Warrants
There is currently no market through which the Warrants may be sold. This may affect the pricing of the Warrants in the secondary market, the transparency and availability of trading prices, the liquidity of the Warrants, and the extent of issuer regulation. There can be no assurance that an active or liquid market for the Warrants will develop following the Offering, or if developed, that such market will be maintained. If an active public market does not develop or is not maintained, purchasers may not be able to resell the Warrants purchased under this Offering.
No Rights as Shareholders
Holders of Warrants will not be entitled to any rights with respect to the Common Shares (including, without limitation, voting rights and rights to receive any dividends or other distributions on the Common Shares), but if a holder of Warrants subsequently exercises its Warrants, such holder will be subject to all changes affecting the Common Shares. Rights with respect to the Common Shares will arise only if and when the Company delivers Common Shares upon the exercising of a Warrant. For example, in the event that an amendment is proposed to the Company’s constating documents requiring shareholder approval and the record date for determining the shareholders of record entitled to vote on the amendment occurs prior to delivery of Common Shares to a holder, such holder will not be entitled to vote on the amendment, although such holder will nevertheless be subject to any changes in the powers or rights of Common Shares that result from such amendment.
Investment Eligibility
There can be no assurance that the Unit Shares, Warrants or Warrant Shares will continue to be “qualified investments” as defined in the Tax Act for trusts governed by Registered Plans or a DPSP. The Tax Act imposes penalties for the acquisition or holding of non-qualified or prohibited investments. See “Eligibility for Investment”.
-53-
Trading Market
The Company cannot assure that a market will continue to develop or be sustained for the Common Shares. If a market does not continue to develop or is not sustained, it may be difficult for purchasers to sell Common Shares at an attractive price or at all. The Company cannot predict the prices at which the Common Shares will trade.
Significant Sales of Common Shares May Have an Adverse Effect on the Market Price of the Common Shares
Significant sales of Common Shares, or the availability of such securities for sale, could adversely affect the prevailing market prices for the Common Shares. A decline in the market prices of the Common Shares could impair the Company’s ability to raise additional capital through the sale of securities should it desire to do so.
Volatile Market Price for the Common Shares
The market price for Common Shares may be volatile and subject to wide fluctuations in response to numerous factors, many of which will be beyond the Company’s control, including, but not limited to the following: (i) actual or anticipated fluctuations in the Company’s quarterly results of operations; (ii) recommendations by securities research analysts; (iii) changes in the economic performance or market valuations of companies in the industry in which the Company will operate; (iv) addition or departure of the Company’s executive officers, directors and other key personnel and consultants; (v) release or expiration of transfer restrictions on outstanding Common Shares; (vi) sales or perceived sales of additional shares; (vii) operating and financial performance that vary from the expectations of management, securities analysts and purchasers; (viii) regulatory changes affecting the Company’s industry generally and its business and operations both domestically and abroad; (ix) announcements of developments and other material events by the Company or its competitors; (x) fluctuations in the costs of vital production materials and services; (xi) changes in global financial markets, global economies, general market conditions, interest rates and pharmaceutical product price volatility which may be impacted by a variety of factors, including but not limited to the current COVID-19 pandemic; (xii) significant acquisitions or business combinations, strategic partnerships, joint ventures or capital commitments by or involving the Company or its competitors; (xiii) operating and share price performance of other companies that purchasers deem comparable to the Company or from a lack of market comparable companies; and (xiv) news reports relating to trends, concerns, technological or competitive developments, regulatory changes and other related issues in the Company’s industry or target markets.
Financial markets have recently experienced significant price and volume fluctuations that have particularly affected the market prices of equity securities of companies and that have often been unrelated to the operating performance, underlying asset values or prospects of such companies (such as the current COVID-19 pandemic). Accordingly, the market price of Common Shares may decline even if the Company’s operating results, underlying asset values or prospects have not changed. Additionally, these factors, as well as other related factors, may cause decreases in asset values that are deemed to be other than temporary, which may result in impairment losses. There can be no assurance that continuing fluctuations in price and volume will not occur. If such increased levels of volatility and market turmoil continue, the Company’s operations could be adversely impacted, and the trading price of Common Shares may be materially adversely affected.
Additional Issuances and Financings May Result in Dilution
Subject to compliance with applicable securities laws and stock exchange rules, the Company’s articles allow it to issue an unlimited number of Common Shares for such consideration and on such terms and conditions as shall be established by the Board of Directors, in many cases, without the approval of the Company’s shareholders. As part of this Offering, and assuming full exercise of Warrants and Compensation Options, the Company may issue up to 61,480,000 Common Shares (or up to 70,702,000 Common Shares if the Over-Allotment Option is exercised in full). Except as described under the heading “Plan of Distribution”, the Company may issue additional Common Shares in subsequent offerings (including through the sale of securities convertible into or exchangeable for Common Shares) and on the exercise of stock options or other securities exercisable for Common Shares. The Company may also issue Common Shares to finance future acquisitions. The Company cannot predict the size of future issuances of Common Shares or the effect that future issuances and sales of Common Shares will have on the market price of the Common Shares. Issuances of a substantial number of additional Common Shares, or the
-54-
perception that such issuances could occur, may adversely affect prevailing market prices for the Common Shares. With any additional issuance of Common Shares, purchasers will suffer dilution to their voting power and the Company may experience dilution in its earnings per share.
Risks Related to the Business
Impact of the COVID-19 Pandemic
Catastrophic events in general can have a material impact on the potential continuity of the business. The Company’s ability to cultivate cannabis and operate dispensaries, among other business activities, may be adversely affected or disrupted as a result. In addition, the Company relies, in part, on third‐party service providers to assist them in managing, monitoring and otherwise carrying out aspects of its business and operations, and the outbreak may affect their ability to devote sufficient time and resources to perform work for the Company. Such events may result in a period of business disruption, reduced operations, any of which could materially affect our business, financial condition and results of operations.
The spread of COVID‐19, which has caused a broad impact globally, may materially affect the Company economically. While the potential economic impact brought by, and the duration of, COVID‐19 may be difficult to assess or predict, a widespread pandemic could result in significant disruption of global financial markets, reducing the Company’s ability to access capital, which could in the future negatively affect our liquidity.
The global outbreak of COVID‐19 continues to evolve rapidly. The extent to which COVID‐19 may impact the Company’s business, operations and financial performance will depend on future developments, including but not limited to, matters such as (a) the duration and/or severity of the outbreak, (b) government policies, restrictions and requirements as they relate to social distancing, forced quarantines and other requirements, (c) non‐ governmental influences or challenges such as the failure of banks and/or (d) any kind of ripple effect caused by the substantial economic damage that can be inflicted on society by a pandemic like COVID‐19 such as lawlessness. The ultimate long‐term impact of COVID‐19 is highly uncertain and cannot be predicted with confidence.
Cash Flow from Operations
The Company had negative operating cash flow for the financial year ended July 31, 2019. The Company cannot guarantee that it will attain or maintain positive cash flow status into the future. To the extent that the Company has negative cash flow in any future period, certain of the proceeds from the Offering may be used to fund such negative cash flow from operating activities in these periods, see “Use of Proceeds”.
No Assurance That Announced Transactions Will Be Completed or That the Company Will Realize Benefits
The Company has announced certain acquisitions and transactions with third parties including, but not limited to, PharmaCo, Avicanna and Critical 39 (the “Proposed Transactions”). Completion of each of the Proposed Transactions will be subject to conditions precedent and the procurement of regulatory, shareholder and other required approvals. There can be no assurance that the Company will complete any or all of the Proposed Transactions.
A number of non-recurring transaction-related costs will be incurred in connection with the Proposed Transactions and with integrating the Proposed Transactions with the business of the Company and achieving desired synergies. Non-recurring transaction costs include, but are not limited to, fees paid to legal, financial and accounting advisors, filing fees and printing costs.
The estimates of benefits associated with the Proposed Transactions reflect estimates and assumptions made by the Company, and it is possible that these estimates and assumptions may not ultimately reflect actual results. In addition, these estimated benefits may not actually be achieved in the timeframe anticipated or at all. If the Company fails to realize anticipated benefits, synergies or revenue enhancements, it could have a material adverse effect on the Company’s business, financial condition and results of operations. There can be no assurance that the realization of efficiencies related to the integration of the Proposed Transactions into the Company’s business,
-55-
will offset the incremental transaction-related costs over time. Any net benefit may not be achieved in the near term, the long term or at all.
No Control Over Operations
The Company may not be directly involved in the ownership or operation of and may have no or limited contractual rights relating to the operations of its current and/or future royalty, equity, debt or other investee entities (collectively, the “Investees”). The Investees will generally have the power to determine the manner in which the business of such Investee is developed, expanded and operated. The interests of the Company and the Investees may not always be aligned. As a result, the cash flows of the Company from royalties, debt instruments or otherwise will be dependent upon the activities of the Investees, which creates the risk that at any time those Investees may: (i) have business interests or targets that are inconsistent with those of the Company; (ii) take action contrary to the Company’s policies or objectives; (iii) be unable or unwilling to fulfill their obligations under their agreements with the Company; or (iv) experience financial, operational or other difficulties, including insolvency, which could limit or suspend an Investee’s ability to perform its obligations under agreements with the Company. There is also the risk that such Investees may not comply with applicable laws, including by operating in jurisdictions where their activities are in breach of the laws of such jurisdictions. There can be no assurance that the Investees involved in the production of cannabis will ultimately meet forecasts or targets. Payments to the Company, in certain instances, will be calculated by the Investees based on reported production, and such payments are subject to, and dependent upon, the adequacy and accuracy of the operators’ production and accounting functions. The Company must rely on the accuracy and timeliness of the public disclosure and other information it receives from the Investees. If the information contains material inaccuracies or omissions, the Company’s ability to accurately forecast or achieve its stated objectives may be materially impaired. Failure to receive the Company’s entitlements pursuant to the agreements it has entered into may have a material adverse effect on the Company.
Compliance With Laws
The Company’s and the Investees’ operations are subject to various laws, regulations and guidelines that may change over time. The Company will endeavour to cause the Investees to comply with all relevant laws, regulations and guidelines at all times. Although some of the Investees are contractually required to comply with laws pursuant to their agreements with the Company, certain Investees are not subject to such requirements, and in any event these contractual obligations do not guarantee compliance by Investees. In the event that an is discovered not to be in compliance with laws, including U.S. laws, the Company may be limited in its recourse against such Investee. In addition, the Investees may not maintain internal policies and procedures adequate to ensure compliance with the various laws, regulations and guidelines to which they are subject. There is also a risk that the Company’s and the Investees’ interpretation of laws, regulations and guidelines, including, but not limited to, various U.S. state regulations and applicable stock exchange rules and regulations, may differ from those of others, including those of government authorities, securities regulators and exchanges, and the Company’s and the Investees’ operations may not be in compliance with such laws, regulations and guidelines. The Company or the Investees, while they may be compliant today, may not be compliant following changes to any laws, regulations or guidelines. In addition, achievement of the Company’s business objectives is contingent, in part, upon compliance with regulatory requirements enacted by governmental authorities and, where necessary, obtaining regulatory approvals. The impact of regulatory compliance regimes, and the impact of any delays in obtaining or failures to obtain regulatory approvals required by the Company or the Investees may significantly delay or impact the development of the Company’s business and operations and could have a material adverse effect on the business, financial condition and results of operations of the Company. In addition, any potential noncompliance could cause the business, financial condition and results of operations of the Company to be adversely affected. Further, any amendment to or replacement of applicable rules and regulations governing the activities of the Company and the Investees may cause adverse effects on the Company’s or the Investees’ business, financial condition and results of operations. The risks to the business of the Company and the Investees associated with any amendment or replacement of applicable regulatory regime the United States could reduce the available market for products or services of the Investee and could materially and adversely affect the business, financial condition and results of operations of the Company.
The Company and the Investees will incur ongoing costs and obligations related to regulatory compliance. Failure to comply with applicable laws and regulations may result in enforcement actions thereunder, including orders
-56-
issued by regulatory or judicial authorities causing operations to cease or be curtailed, and may include corrective measures requiring capital expenditures or remedial actions. Parties may be liable for civil or criminal fines or penalties imposed for violations of applicable laws or regulations. Amendments to current laws, regulations and permitting requirements, court rulings or more stringent application of existing laws or regulations, may have a material adverse impact on the Company and/or the Investees, resulting in increased capital expenditures or production costs, reduced levels of cannabis production or abandonment or delays in the development of facilities, or other significant changes in the Company’s and/or the Investees’ business plans, which could have a material adverse effect on the business, financial condition and results of operations of the Company and/or the Investees.
The introduction of new tax laws, regulations or rules, or changes to, or differing interpretation of, or application of, existing tax laws, regulations or rules in any of the countries in which the Company may operate could result in an increase in the Company’s taxes, or other governmental charges, duties or impositions. No assurance can be given that new tax laws, regulations or rules will not be enacted or that existing tax laws, regulations or rules will not be changed, interpreted or applied in a manner which could result in the Company’s profits being subject to additional taxation or which could otherwise have a material adverse effect on the Company.
Due to the complexity and nature of the Company’s operations, various legal and tax proceedings may be in progress from time to time. If the Company is unable to resolve any of these proceedings favourably, there may be a material adverse effect on the Company.
Business Strategy
The Company’s business strategy involves constantly seeking new opportunities in the U.S. legal cannabis industry. In the pursuit and execution of such opportunities, the Company may fail to select appropriate investment candidates and/or fail to negotiate beneficial or advantageous contractual arrangements. The Company cannot provide assurance that it can complete any investment or business arrangement that it pursues or is pursuing, on favourable terms, or that any investments or business arrangements completed will ultimately benefit the Company.
In the event that the Company chooses to raise debt capital to finance investments, the Company’s leverage will be increased. In addition, if the Company chooses to complete an equity financing, shareholders may suffer dilution.
Risks Inherent in Strategic Alliances and Investments
The Company may enter into further strategic alliances with third parties that it believes will complement or augment its existing business. The Company’s ability to form strategic alliances is dependent upon, and may be limited by, the availability of suitable candidates and capital. In addition, strategic alliances could present unforeseen integration obstacles or costs, may not enhance the Company’s business, and/or may involve risks that could adversely affect the Company, including significant amounts of management time that may be diverted from operations to pursue and complete such transactions or maintain such strategic alliances. Future strategic alliances could result in the incurrence of additional debt, costs and/or contingent liabilities, and there can be no assurance that future strategic alliances will achieve the expected benefits to the Company’s business or that the Company will be able to consummate future strategic alliances on satisfactory terms, or at all.
While the Company conducted due diligence with respect to the Investees, there are risks inherent in any investment. Specifically, there could be unknown or undisclosed risks or liabilities of the Investees for which the Company is not sufficiently indemnified or at all. Any such unknown or undisclosed risks or liabilities could materially and adversely affect the Company’s financial performance and results of operations. It is currently anticipated that the investments will be accretive; however, the performance of such an investment may be materially different. The Company could encounter additional transaction and enforcement-related costs, and may fail to realize all of the potential benefits from its investments. Any of the foregoing risks and uncertainties could have a material adverse effect on the Company’s business, financial condition and results of operations.
Risks Associated With Divestment and Restructuring
In certain circumstances, the Company may decide, or be required, to divest its investment in certain Investees. In particular, if any of the Investees violate any applicable laws and regulations, including U.S. federal law, the
-57-
Company may be required to divest its interest in such Investee or risk significant fines, penalties, administrative sanctions, convictions, settlements or delisting from the CSE. For instance, if the Company determines that the operations of an Investee are not compliant with U.S. laws or the policies of the CSE, the Company will use its commercially reasonable efforts to divest of its interest in the Investee in the event that it cannot restructure its holdings. There is no assurance that these divestitures will be completed on terms favourable to the Company, or at all. Any opportunities resulting from these divestitures, and the anticipated effects of these divestitures on the Company may never be realized, or may not be realized to the extent the Company anticipates. Moreover, there is no guarantee that the Company will realize gains on its investments based on the fair value of underlying financial assets. In pursuit of opportunities to dispose of any of the Company’s investments, third parties may not ascribe similar value to such investments as the Company and the Company may not be able to obtain the value it ascribes to such investments. Not all of the Company’s investments are liquid, and such investments may be difficult to dispose of and subject to illiquidity discounts on divestiture. The securities of the Investees are not generally listed on any stock exchange. Any required divestiture or an actual or perceived violation of applicable laws or regulations by the Company or any Investee could have a material adverse effect on the Company, including its reputation and ability to conduct business, its holdings (directly or indirectly) in the Investees, the listing of its securities on applicable stock exchanges, its financial position, operating results, profitability or liquidity or the market price of the Common Shares. In addition, it is difficult for the Company to estimate the time or resources that may be required for the investigation of any such matter or its final resolution because, in part, the time and resources that may be needed are dependent on the nature and extent of any information requested by the applicable authorities involved, and such time or resources could be substantial.
If the Company decides, or is required, to restructure its investments in any Investees to remain in compliance with laws or stock exchange requirements, such restructuring could result in the write-down of the value of the Company’s investments, which could have a material adverse effect on the Company’s business, financial condition and results of operations.
Investors in the Company May Be Subject to Entry Bans into the United States
Cannabis remains illegal under U.S. federal law. Individuals employed at or investing in cannabis companies could face detention, denial of entry or lifetime bans from the U.S. for their business associations with cannabis businesses. Entry to the U.S. is granted at the sole discretion of Customs and Border Protection (“CBP”) officers on duty, and these officers have wide latitude to ask questions to determine the admissibility of a foreign national. The government of Canada has started warning travelers that previous use of cannabis, or any substance prohibited by U.S. federal laws, could result in denial of entry to the U.S. Business or financial involvement in the cannabis industry in Canada or in the U.S. could also be reason enough for CBP officers to deny entry. On September 21, 2018, CBP released a statement outlining its position with respect to enforcement of the laws of the U.S. It stated that Canada’s legalization of cannabis will not change CBP enforcement of U.S. laws regarding controlled substances and because cannabis continues to be a controlled substance under U.S. law, working in or facilitating the proliferation of the cannabis industry in U.S. states or Canada may affect admissibility to the U.S. On October 9, 2018, CBP released an additional statement regarding the admissibility of Canadian citizens working in the U.S. legal cannabis industry. CBP stated that a Canadian citizen working in or facilitating the proliferation of the U.S. legal cannabis industry in Canada coming into the U.S. for reasons unrelated to the cannabis industry will generally be admissible to the U.S.; however, if such person is found to be coming into the U.S. for reasons related to the cannabis industry, such person may be deemed inadmissible. Employees, directors, officers, managers and investors of companies involved in business activities related to cannabis in the U.S. or Canada (such as the Company), who are not U.S. citizens, face the risk of being barred from entry into the U.S. for life.
Difficulty to Forecast
The Company will need to rely largely on its own market research to forecast industry trends and statistics as detailed forecasts are, with certain exceptions, not generally available from other sources at this early stage of the cannabis industry. A failure in the demand for the products of certain Investees to materialize as a result of competition, technological change, change in the regulatory or legal landscape or other factors could have a material adverse effect on the business, financial condition and results of operations of the Company.
-58-
Cannabis Prices
The price of the Common Shares and the Company’s financial results may be significantly and adversely affected by a decline in the price of cannabis. There is currently not an established market price for cannabis and the price of cannabis is affected by numerous factors beyond the Company’s control. Any price decline may have a material adverse effect on the Company.
The profitability of the Company’s interests under agreements with certain Investees is directly related to the price of cannabis. The Company’s operating income may be sensitive to changes in the price of cannabis and the overall condition of the cannabis industry, as its operating income will be derived in part from royalty payments. In addition, the value of the Company’s investments in the Investees may be affected as a result of changes in the prevailing market price of cannabis, which may have a material adverse effect on the ability of the Investees to generate positive cash flow or earnings.
Reputational Risk
The Company believes that the cannabis industry is highly dependent upon consumer perception regarding the safety, efficacy and quality of the cannabis produced. Consumer perception can be significantly influenced by scientific research or findings, regulatory proceedings, litigation, media attention and other publicity regarding the consumption of cannabis products. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favourable to the cannabis market or any particular product, or consistent with currently held views. Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that are perceived as less favourable than, or that question, earlier research reports, findings or publicity could have a material adverse effect on the cannabis industry and demand for its products and services, which could affect the business, financial condition and results of operations and cash flows of the Company. The Company’s dependence upon consumer perception means that adverse scientific research reports, findings, regulatory proceedings, litigation, media attention or other publicity, whether or not accurate or with merit, could have a material adverse effect on the Company, the business, financial condition, results of operations and cash flows of the Company. Further, adverse publicity, reports or other media attention regarding the safety, efficacy and quality of cannabis in general, or the Investees’ products specifically, or associating the consumption of cannabis with illness or other negative effects or events, could have a material adverse effect. Such adverse publicity reports or other media attention could arise even if the adverse effects associated with such products resulted from consumers’ failure to consume such products legally, appropriately, or as directed.
In addition, parties outside of the cannabis industry with which the Company does business may perceive that they are exposed to reputational risk as a result of the Company’s cannabis related business activities. For example, the Company could receive a notification from a financial institution advising it that they would no longer maintain banking relationships with those in the cannabis industry. The Company may in the future have difficulty establishing or maintaining bank accounts or other business relationships that it needs to operate its business. Failure to establish or maintain business relationships could have a material adverse effect on the Company.
Equity Price Risk
The Company may be exposed to equity price risk as a result of holding long-term investments in cannabis companies. Just as investing in the Company carries inherent risks, such as those set out in this Prospectus, the Company faces similar inherent risks by investing in other cannabis companies, and accordingly may be exposed to the risks associated with owning equity securities in the Investees.
Anti-Bribery Law Violations
The Company’s business is subject to laws which generally prohibit companies and employees from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. In addition, the Company is or will be subject to the anti-bribery laws of any other countries in which it conducts business now or in the future. The Company’s employees or other agents may, without its knowledge and despite its efforts, engage in prohibited conduct under the Company’s policies and procedures and anti-bribery laws for
-59-
which the Company may be held responsible. The Company’s policies mandate compliance with these anti- corruption and anti-bribery laws. However, there can be no assurance that the Company’s internal control policies and procedures will always protect it from recklessness, fraudulent behaviour, dishonesty or other inappropriate acts committed by its affiliates, employees, contractors or agents. If the Company’s employees or other agents are found to have engaged in such practices, the Company could suffer severe penalties and other consequences that may have a material adverse effect on its business, financial condition and results of operations.
Modifications to Investment Policy
Although the Company has adopted an investment policy regarding the types of interests that it seeks to invest in, acquire or otherwise enter into a strategic relationship with, the Company does not currently have any limitations on investments (including, among other things, geographic location, diversity, number or concentration of investments, timing of investments or the specific focus of investments). Further, senior management of the Company may propose, and the Board of Directors may use their discretion to approve, modifications or exceptions to the investment policy in the future for valid business reasons, subject to shareholder approval in certain circumstances as set forth in the investment policy. If the Company modifies its investment policy or makes exceptions to it, such modifications or exceptions may have the effect of materially increasing the risk profile of an investment in the Company. In addition, the Company’s investment portfolio may be highly concentrated in a small number of investments, which may materially adversely affect the Company’s financial position if any of those investments do not perform as anticipated.
Passive Foreign Investment Company Status
Under United States federal income tax laws, if a company is (or for any past period was) a PFIC, it could have adverse United States federal income tax consequences to U.S. shareholders even if the company is no longer a PFIC. The determination of whether the Company is a PFIC is a factual determination made annually based on all the facts and circumstances and thus is subject to change. Furthermore, the principles and methodology used in determining whether a company is a PFIC are subject to interpretation.
If the Company is deemed to be a PFIC, U.S. holders would be subject to adverse U.S. federal income tax consequences such as the ineligibility for any preferred tax rates on capital gains, the ineligibility for actual or deemed dividends, interest charges on certain taxes treated as deferred, and additional reporting requirements under U.S. federal income tax laws or regulations. Whether or not U.S. holders make a timely qualified electing fund (or QEF) election or mark-to-market election may affect the U.S. federal income tax consequences to U.S. holders with respect to the acquisition, ownership, and disposition of Common Shares and any distributions such U.S. holders may receive.
Risks Relating to RWB Platinum Vape and the Investees
In addition to the risk factors that may impact the business, operations and financial condition of the Company and the Investees noted above, the following supplemental risk factors may directly impact the business, operations and financial condition of RWB Platinum Vape and certain Investees and, accordingly, may have an indirect material adverse effect on the Company.
Reliance on Licences
The Company will be dependent on the licences or ability to obtain a licence of RWB Platinum Vape and certain Investees, which are subject to ongoing compliance and reporting requirements. Failure to comply with the requirements of these licences or any failure to obtain or maintain those licences could have a material adverse impact on the business, financial condition and operating results of the Company. There can be no guarantee that a licence will be issued, extended or renewed or, if issued, extended or renewed, that they will be issued, extended or renewed on terms that are favourable to such licensee and the Company. Should a licence not be issued, extended or renewed or should it be issued or renewed on terms that are less favourable to the respective licensee and the Company than anticipated, the business, financial condition and results of the operations of the Company could be materially adversely affected.
-60-
Failure to Obtain Necessary Licences
The abilities of RWB Platinum Vape and certain Investees to grow, store and sell cannabis in the United Sates are dependent on securing and maintaining the appropriate licences with the applicable regulatory authorities. Failure to comply with the requirements of any license application or failure to obtain and maintain the appropriate licences with the relevant authorities could have a material adverse impact on the business, financial condition and results of operations of RWB Platinum Vape, the Investee and the Company. There can be no guarantees that regulatory authorities will issue the required licences.
Reliance on Facilities
The facilities used by RWB Platinum Vape and certain Investees could be subject to adverse changes or developments, including but not limited to a breach of security, which could have a material and adverse effect on the Company’s business, financial condition and prospects. Any breach of the security measures and other facility requirements, including any failure to comply with recommendations or requirements arising from inspections by regulatory authorities, could also have an impact on such Investee’s ability to continue operating under their licences or the prospect of renewing their licences, which may have an adverse effect on the Company.
Governmental Regulations
Cannabis operations are subject to extensive laws and regulations. The costs of compliance with such laws and regulations are significant. It is possible that the costs and delays associated with compliance with such laws and regulations could become such that RWB Platinum Vape and/or the Investees would not continue to develop or operate their businesses. Moreover, it is possible that future regulatory developments could result in substantial costs and liabilities for RWB Platinum Vape and/or the Investees in the future such that they would not continue to develop or operate their business. In addition, RWB Platinum Vape and the Investees are subject to various laws, regulations and guidelines, including, but not limited to the applicable stock exchange rules and regulations.
Operating Risks
Cannabis operations generally involve a high degree of risk. RWB Platinum Vape and the Investees are subject to the hazards and risks normally encountered in the cannabis industry. Should any of these risks or hazards affect RWB Platinum Vape or one of the Investees, it may (i) cause the cost of development or production to increase to a point where it would no longer be economic to produce cannabis, (ii) cause delays or stoppage of operations, (iii) cause personal injury or death and related legal liability, or (iv) result in the loss of insurance coverage. The occurrence of any of these risks or hazards could have a material adverse effect on the Company and the price of the Common Shares.
The production of cannabis involves significant risks. In the United States, not all applicants for a licence ultimately receive a licence to produce and sell cannabis. Major expenditures may be required in pursuit of a licence and it is impossible to ensure that the expenditures will result in receipt of a licence and a profitable operation. There can be no assurances that RWB Platinum Vape or any of the Investees will obtain and maintain a licence to produce and sell cannabis and be brought into a state of commercial production.
Increased Operational, Regulatory and Other Risks
RWB Platinum Vape and/or an Investee may in the future expand into other geographic areas, product categories or market segments, which could increase the Investee’s operational, regulatory, compliance, reputational and foreign exchange rate risks. The failure of RWB Platinum Vape or the Investee’s operating infrastructure to support such expansion could result in operational failures and regulatory fines or sanctions. Future international expansion could require RWB Platinum Vape and/or the Investees to incur a number of upfront expenses, including those associated with obtaining regulatory approvals, as well as additional ongoing expenses, including those associated with infrastructure, staff and regulatory compliance. RWB Platinum Vape and/or the Investees may not be able to successfully identify suitable acquisition, investment and/or expansion opportunities or integrate such operations successfully with RWB Platinum Vape’s and/or the Investees’ existing operations.
-61-
Access to Capital for Investees
Investees that operate in the U.S. may have difficulty accessing the services of banks and processing credit card payments, which may make it difficult for these Investees to operate. In February 2014, the FinCEN issued guidance with respect to financial institutions providing banking services to cannabis business, including burdensome due diligence expectations and reporting requirements. This guidance does not provide any safe harbors or legal defences from examination or regulatory or criminal enforcement actions by the Department of Justice, FinCEN or other federal regulators. Thus, most banks and other financial institutions do not appear to be comfortable providing banking services to cannabis-related businesses. In addition to the foregoing, banks may refuse to process debit card payments and credit card companies generally refuse to process credit card payments for cannabis-related businesses. As a result, Investees that operate in the U.S. may have limited or no access to banking or other financial services in the U.S., and may have to operate their businesses on an all-cash basis. The inability or limitation on certain Investees’ ability to open or maintain bank accounts in the U.S., obtain other banking services and/or accept credit card and debit card payments may make it difficult for such Investees to operate and conduct their businesses as planned in the United States.
Enforceability of Contracts
Certain contracts of RWB Platinum Vape and/or Investees may involve cannabis or cannabis-related businesses and other activities that are not legal under U.S. federal law. In some jurisdictions, RWB Platinum Vape and/or such Investees may face difficulties in enforcing their contracts in U.S. federal and certain state courts.
Lack of Access to U.S. Bankruptcy Protections
As cannabis is a Schedule I substance under the Controlled Substances Act, many courts have denied cannabis businesses federal bankruptcy protections, making it difficult for lenders to recover their investments in the cannabis industry in the event of a bankruptcy. If the Company or one of the Investees were to experience a bankruptcy, there is no guarantee that U.S. federal bankruptcy protections would be available to the Company or such Investee, which would have a material adverse effect on the Company.
Production Forecasts
The Company will prepare estimates and forecasts of future attributable production from certain Investees and will rely on public disclosure and other information it receives from the owners, operators and independent experts to prepare such estimates. Such information will be necessarily imprecise because it will depend upon significant judgment. In addition, the Company will rely largely on its own market research to forecast sales as detailed forecasts are not generally obtainable from other sources at this early stage of the cannabis industry. These production estimates and projections will be based upon existing plans and other assumptions which change from time to time, and over which the Company will have no control, including the costs of production, the operators’ ability to sustain and increase production levels, the sufficiency of infrastructure, the performance of personnel and equipment, the ability to maintain and obtain licences and permits and compliance with existing and future laws and regulations. Any such information is forward-looking and no assurance can be given that such production estimates and projections will be achieved. Actual attributable production may vary from the Company’s estimates for a variety of reasons and may result in the failure to achieve the production forecasts currently anticipated. If the Company’s forecasts prove to be incorrect, it may have a material adverse effect on the Company.
Competitive Conditions
The Investees will face intense competition from other companies, some of which have longer operating histories as well as more financial resources, production capacity and marketing experience than the Investees. Increased competition by larger and better financed competitors could materially and adversely affect the business, financial condition and results of operations of the Investees, including the Investees’ ability to source starting materials, retain qualified employees, enter into supply agreements, develop retail sales channels and obtain a share of the overall cannabis market. Accordingly, the business, financial conditions and results of operations of the Company would also be similarly affected.
-62-
Customer Acquisitions
The Company’s success depends, in part, on the ability of RWB Platinum Vape and the Investees to attract and retain customers. There are many factors which could impact the ability to attract and retain customers, including but not limited to the ability of RWB Platinum Vape and/or certain Investees to continually produce desirable and effective product, the successful implementation of customer-acquisition plans and the continued growth in the aggregate number of customers. The failure to acquire and retain customers would have a material adverse effect on the business, operating results and financial condition of RWB Platinum Vape and/or certain Investees, which could have a materially adverse effect on the Company.
Risks Inherent in an Agricultural Business
The business of RWB Platinum Vape and certain of the Investees involves the growing of cannabis. Cannabis is an agricultural product. As such, the business is subject to the risks inherent in the agricultural business, such as insects, plant diseases and similar agricultural risks. Weather conditions, which can vary substantially from year to year, may have a significant impact on the size and quality of the harvest of the crops processed and sold by the Investees. Significant increases or decreases in the total harvest will impact the Company’s profits realized on sales of RWB Platinum Vape and/or the Investees’ products and, consequently, the results of the Company’s operations. High degrees of quality variance can also affect processing velocity and capacity utilization, as the processes required to potentially upgrade lower or more variable quality product can slow overall processing times. There can be no assurance that natural elements will not have a material adverse effect on the production of products by RWB Platinum Vape and/or certain Investees, which may have a material adverse effect on the Company.
Wholesale Price Volatility
The cannabis industry is a margin-based business in which gross profits depend on the excess of sales prices over costs. Consequently, profitability is sensitive to fluctuations in wholesale and retail prices caused by changes in supply (which itself depends on other factors such as weather, fuel, equipment and labour costs, shipping costs, economic situation and demand), taxes, government programs and policies for the cannabis industry (including price controls and wholesale price restrictions that may be imposed by provincial agencies responsible for the sale of cannabis), and other market conditions, all of which are factors beyond the control of the Company and the Investees.
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
The following is, as of the date hereof, a summary of the principal Canadian federal income tax considerations pursuant to the Tax Act generally applicable to a purchaser who acquires an Offered Unit pursuant to this Offering. For purposes of this summary, references to Common Shares include Unit Shares and Warrant Shares unless otherwise indicated.
This summary applies only to a purchaser who is a beneficial owner of Unit Shares and Warrants acquired pursuant to this Offering and who, for the purposes of the Tax Act, and at all relevant times: (i) deals at arm’s length and is not affiliated with the Company or the Underwriters; and (ii) acquires and holds the Unit Shares, Warrants and any Warrant Shares acquired on the exercise of the Warrants as capital property (a “Holder”). Generally, the Common Shares and Warrants will be considered to be capital property to a Holder unless the Holder holds such securities in the course of carrying on a business of trading or dealing in securities or has acquired them in one or more transactions considered to be an adventure or concern in the nature of trade.
This summary does not apply to a Holder (a) that is a “financial institution” for purposes of the mark-to-market rules contained in the Tax Act; (b) that is a “specified financial institution”, as defined in the Tax Act; (c) an interest in which is a “tax shelter investment”, as defined in the Tax Act; (d) that has made a functional currency reporting election under the Tax Act; (e) that has entered or will enter into a “derivative forward agreement” or a “synthetic disposition arrangement”, as defined in the Tax Act, with respect to the Common Shares or the Warrants; or (f) that receives dividends on the Common Shares under or as part of a “dividend rental arrangement”, as defined in the Tax Act. Such Holders should consult with their own tax advisors with respect to an investment in Offered
-63-
Units. Additional considerations, not discussed herein, may be applicable to a Holder that is a corporation resident in Canada, and is, or becomes as part of a transaction or event or series of transactions or events that includes the acquisition of the Offered Units, controlled by a non-resident corporation (or pursuant to the Tax Proposals, a non-resident person a group of persons (comprised of any combination of non-resident corporations, non-resident individuals or non-resident trusts) that do not deal at arm’s length) for purposes of the “foreign affiliate dumping” rules in section 212.3 of the Tax Act. Such Holders should consult their tax advisors with respect to the consequences of acquiring Offered Units.
This summary is based upon the current provisions of the Tax Act in force as of the date hereof, counsel’s understanding of the current published administrative policies and assessing practices of the Canada Revenue Agency (the “CRA”) published by it in writing prior to the date hereof. This summary takes into account all specific proposals to amend the Tax Act publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Tax Proposals”) and assumes that the Tax Proposals will be enacted substantially as proposed; however, no assurance can be given that the Tax Proposals will be enacted as proposed or at all. This summary does not otherwise take into account or anticipate any changes in law or the CRA’s administrative policies or assessing practices, whether by legislative, governmental, administrative or judicial decision or action, nor does it take into account any provincial, territorial or foreign income tax legislation or considerations, which considerations may differ significantly from the Canadian federal income tax considerations discussed in this summary.
This summary is of a general nature only, is not exhaustive of all possible Canadian federal income tax considerations and is not intended to be, nor should it be construed to be, legal or tax advice to any particular Holder. Accordingly, Holders should consult their own tax advisors with respect to their particular circumstances.
Allocation of Cost
A Holder who acquires Offered Units pursuant to this Offering will be required to allocate the purchase price paid for each Offered Unit on a reasonable basis between the Unit Share and the Warrant comprising each Offered Unit in order to determine their respective costs to such Holder for the purposes of the Tax Act.
For its purposes, the Company has advised counsel that, of the $0.75 subscription price for each Offered Unit, it intends to allocate $0.67 to each Unit Share and $0.08 to each Warrant. Although the Company believes that this allocation is reasonable, it is not binding on the CRA or on a Holder, and the CRA may not be in agreement with such allocation. Counsel express no opinion with respect to such allocation.
Adjusted Cost Base of a Unit Share
The adjusted cost base to a Holder of each Unit Share comprising a part of an Offered Unit acquired pursuant to this Offering will be determined by averaging the cost of such Unit Share with the adjusted cost base to such Holder of all other Common Shares (if any) held by the Holder as capital property immediately prior to the acquisition.
Exercise of Warrants
No gain or loss will be realized by a Holder of a Warrant upon the exercise of a Warrant to acquire a Warrant Share. When a Warrant is exercised, the Holder’s cost of the Warrant Share acquired thereby will be equal to the adjusted cost base of the Warrant to such Holder, plus the amount paid on the exercise of the Warrant. For the purpose of computing the adjusted cost base to a Holder of each Warrant Share acquired on the exercise of a Warrant, the cost of such Warrant Share must be averaged with the adjusted cost base to such Holder of all other Common Shares (if any) held by the Holder as capital property immediately prior to the exercise of the Warrant.
Holders Resident in Canada
The following section of this summary is generally applicable to a Holder who, for purposes of the Tax Act, is or is deemed to be resident in Canada at all relevant times (a “Resident Holder”). A Resident Holder whose Common
-64-
Shares might not otherwise qualify as capital property, may in certain circumstances, make an irrevocable election permitted by subsection 39(4) of the Tax Act to deem the Common Shares, and every other “Canadian security” (as defined in the Tax Act), held by such Resident Holder in a taxation year of the election and all subsequent taxation years to be capital property. This election does not apply to Warrants. Resident Holders should consult with their own tax advisors regarding this election.
Expiry of Warrants
In the event of the expiry of an unexercised Warrant, a Resident Holder will generally realize a capital loss equal to the Resident Holder’s adjusted cost base of such Warrant. The tax treatment of capital gains and capital losses is discussed in greater detail below under the subheading “Capital Gains and Capital Losses”.
Dividends
A Resident Holder will be required to include in computing its income for a taxation year any taxable dividends received or deemed to be received on the Common Shares. In the case of a Resident Holder that is an individual (other than certain trusts), such dividends will be subject to the gross-up and dividend tax credit rules applicable to taxable dividends received from “taxable Canadian corporations” (as defined in the Tax Act). Taxable dividends received from a taxable Canadian corporation which are designated by such corporation as “eligible dividends” will be subject to an enhanced gross-up and dividend tax credit regime in accordance with the rules in the Tax Act.
In the case of a Resident Holder that is a corporation, the amount of any such taxable dividends that is included in its income for a taxation year will generally be deductible in computing its taxable income for that taxation year.
A Resident Holder that is a “private corporation” or a “subject corporation”, as defined in the Tax Act, may be liable to pay an additional tax under Part IV of the Tax Act (which may be refundable in certain circumstances) on dividends received or deemed to be received on the Common Shares to the extent such dividends are deductible in computing the Resident Holder’s taxable income for the taxation year.
In certain circumstances, subsection 55(2) of the Tax Act will treat a taxable dividend received or deemed to be received by a Resident Holder that is a corporation as proceeds of disposition or a capital gain. Resident Holders that are corporations should consult their own tax advisors having regard to their own circumstances.
Dispositions of Common Shares and Warrants
A Resident Holder who disposes of or is deemed to have disposed of a Common Share or Warrant (other than on the exercise of a Warrant) will generally realize a capital gain (or capital loss) in the taxation year of the disposition equal to the amount by which the proceeds of disposition, net of any reasonable costs of disposition, are greater (or are less) than the adjusted cost base to the Resident Holder of the Common Share or Warrant immediately before the disposition or deemed disposition. The tax treatment of capital gains and capital losses is discussed in greater detail below under the subheading “Capital Gains and Capital Losses”.
Capital Gains and Capital Losses
A Resident Holder will generally be required to include in computing its income for the taxation year, one-half of the amount of any capital gain (a “taxable capital gain”) realized in such year. Subject to and in accordance with the provisions of the Tax Act, a Resident Holder will be required to deduct one-half of the amount of any capital loss (an “allowable capital loss”) against taxable capital gains realized in the taxation year. Allowable capital losses in excess of taxable capital gains for the taxation year of disposition may be carried back and deducted in any of the three preceding taxation years or carried forward and deducted in any subsequent taxation year against net taxable capital gains realized in such years, to the extent and under the circumstances specified in the Tax Act.
The amount of any capital loss realized on the disposition or deemed disposition of a Common Share by a Resident Holder that is a corporation may, in certain circumstances, be reduced by the amount of dividends received or deemed to have been received by it on such shares or on shares substituted therefor to the extent and under the
-65-
circumstances specified in the Tax Act. Similar rules may apply where a Resident Holder that is a corporation is a member of a partnership or a beneficiary of a trust that owns Common Shares, directly or indirectly, through a partnership or trust. Resident Holders to whom these rules may be relevant should consult their own tax advisors.
A Resident Holder that is throughout the relevant taxation year a “Canadian-controlled private corporation” (as defined in the Tax Act) may be liable to pay an additional tax on its “aggregate investment income” (as defined in the Tax Act) for the year, which will include taxable capital gains, which may be refundable in certain circumstances.
Minimum Tax
In general terms, a Resident Holder that is an individual or a trust (other than certain specified trusts) that receives or is deemed to have received taxable dividends on the Common Shares or realizes a capital gain on the disposition or deemed disposition of Common Shares or Warrants may be liable for alternative minimum tax under the Tax Act. Resident Holders that are individuals should consult their own tax advisors in this regard.
Holders Not Resident in Canada
This portion of the summary is generally applicable to a Holder who, at all relevant times, for purposes of the Tax Act: (i) is not, and is not deemed to be, resident in Canada; and (ii) does not use or hold the Common Shares or Warrants in connection with carrying on a business in Canada (“Non-Resident Holder”). This summary does not apply to a Holder that carries on, or is deemed to carry on, an insurance business in Canada and elsewhere or that is an “authorized foreign bank” (as defined in the Tax Act) and such Holders should consult their own tax advisors.
Dividends
Dividends paid or credited or deemed under the Tax Act to be paid or credited by the Company to a Non-Resident Holder on the Common Shares will generally be subject to Canadian withholding tax at the rate of 25% on the gross amount of such dividend, unless such rate is reduced by the terms of an applicable tax treaty or convention. Under the Canada-United States Tax Convention (1980), as amended (the “Treaty”), the rate of withholding tax on dividends paid or credited to a Non-Resident Holder who is resident in the U.S. for purposes of the Treaty and fully entitled to benefits under the Treaty (a “U.S. Holder”) is generally limited to 15% of the gross amount of the dividend (or 5% in the case of a U.S. Holder that is a company beneficially owning at least 10% of the Company’s voting shares).
Dispositions of Common Shares and Warrants
A Non-Resident Holder will not be subject to tax under the Tax Act in respect of any capital gain realized on a disposition or deemed disposition of a Common Share or Warrant unless the Common Share or Warrant (as applicable) is, or is deemed to be, “taxable Canadian property” of the Non-Resident Holder for the purposes of the Tax Act and the Non-Resident Holder is not entitled to an exemption pursuant to the terms of an applicable tax treaty or convention.
Provided that the Common Shares are listed on a “designated stock exchange” for purposes of the Tax Act (which currently includes the CSE), at the time of disposition, the Common Shares and Warrants will generally not constitute taxable Canadian property of a Non-Resident Holder at that time, unless at any time during the 60 month period immediately preceding the disposition, (i) at least 25% of the issued shares of any class or series of the capital stock of the Company were owned by or belonged to one or any combination of (a) the Non-Resident Holder, (b) persons with whom the Non-Resident Holder did not deal at arm’s length, and (c) partnerships in which the Non-Resident Holder or a person described in (b) held a membership interest directly or indirectly through one or more partnerships; and (ii) at such time, more than 50% of the fair market value of such shares was derived, directly or indirectly, from any combination of real or immovable property situated in Canada, “Canadian resource property” (as defined in the Tax Act), “timber resource property” (as defined in the Tax Act), or options in respect of, interests in, or for civil law rights in such properties, whether or not such property exists. The Common Shares
-66-
and Warrants may also be deemed to be taxable Canadian property to a Non-Resident Holder for purposes of the
Tax Act in certain circumstances.
In cases where a Non-Resident Holder disposes (or is deemed to have disposed) of a Common Share or Warrant that is taxable Canadian property to that Non-Resident Holder, and the Non-Resident Holder is not entitled to an exemption under an applicable income tax convention, the consequences described above under the headings “Holders Resident in Canada — Dispositions of Common Shares and Warrants” and “— Capital Gains and Capital Losses” will generally be applicable to such disposition. Such Non-Resident Holders should consult their own tax advisors.
STATUTORY RIGHTS OF WITHDRAWAL AND RESCISSION
Securities legislation in certain of the provinces of Canada provides purchasers with the right to withdraw from an agreement to purchase securities. This right may be exercised within two business days after receipt or deemed receipt of a prospectus and any amendment thereto. In several of the provinces, the securities legislation further provides a purchaser with remedies for rescission or, in some jurisdictions, revisions of the price or damages if the prospectus and any amendment contains a misrepresentation or is not delivered to the purchaser, provided that the remedies for rescission, revisions of the price or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of these rights or consult with a legal adviser.
In an offering comprising warrants (including the Warrants underlying the Offered Units and the Over-Allotment Units), purchasers are cautioned that the statutory right of action for damages for a misrepresentation contained in the prospectus is limited, in certain provincial securities legislation, to the price at which the warrants (including the Warrants comprising part of the Offered Units and the Over-Allotment Units) are offered to the public under the prospectus offering. This means that, under the securities legislation of certain provinces, if the purchaser pays additional amounts upon conversion, exchange or exercise of the security, those amounts may not be recoverable under the statutory right of action for damages that applies in those provinces. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of this right of action for damages or consult with a legal adviser.
INTEREST OF EXPERTS
Certain legal matters in connection with this Offering will be passed upon on behalf of the Company by Gowling WLG (Canada) LLP, and on behalf of the Underwriters by Borden Ladner Gervais LLP. As at the date hereof, the partners and associates of Gowling WLG (Canada) LLP and Borden Ladner Gervais LLP each as a group, beneficially own, directly and indirectly, in the aggregate, less than one percent of the Common Shares.
The audited consolidated financial statements in respect of MichiCann and the notes thereto as at and for the years ended December 31, 2019 and 2018, together with the auditor’s report thereon were prepared by Smythe LLP, Chartered Professional Accountants (“Smythe”), as auditors of MichiCann. Smythe has advised that it is independent with respect to MichiCann and the Company within the meaning of the relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulations.
The audited consolidated financial statements in respect of the Company and the notes thereto as at and for the years ended July 31, 2019 and 2018, together with the auditor’s report thereon were prepared by Manning Elliott LLP (“Manning Elliott”), as auditors of the Company. Manning Elliott has advised that it is independent with respect to the Company within the meaning of the relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulations.
AUDITORS, TRANSFER AGENT AND REGISTRAR
The Company’s auditors are Smythe, located at 1700-475 Howe St., Vancouver, BC V6C 2B3. Smythe has confirmed with respect to the Company, that they are independent within the meaning of the relevant rules and
-67-
related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulations.
On August 20, 2020, Manning Elliott, the former auditors of the Company, resigned at the request of the Board of Directors. The Board of Directors appointed Smythe as auditors of the Company effective August 20, 2020 to fill the vacancy created thereby. Copies of the Company’s Notice of Change of Auditor and each of the letters provided by Manning Elliott and Smythe in response (collectively, the “Reporting Package”) is filed on SEDAR. The Reporting Package has been reviewed and approved by the Board of Directors of the Company.
The transfer agent and registrar for the Common Shares is National Securities Administrators Ltd. at its principal offices in Vancouver.
-68-
CERTIFICATE OF THE COMPANY
September 18, 2020
This short form prospectus, together with the documents incorporated by reference, constitutes full, true and plain disclosure of all material facts relating to the securities offered by this short form prospectus as required by the securities legislation of each of the provinces of Canada, except Québec.
(signed) “Brad Rogers” (signed) “Johannes van der Linde”
Chief Executive Officer Chief Financial Officer
On behalf of the Board of Directors
(signed) “Michael Marchese” (signed) “Brendan Purdy”
Director Director
C-1
CERTIFICATE OF THE UNDERWRITERS
September 18, 2020
To the best of our knowledge, information and belief, this short form prospectus, together with the documents incorporated by reference, constitutes full, true and plain disclosure of all material facts relating to the securities offered by this short form prospectus as required by the securities legislation of each of the provinces of Canada, except Québec.
C-2




